Study on dispersion of abrasive particles in electro Fenton CMP slurry and design of green polishing fluid in neutral environment
-
摘要: 芬顿反应是一种能产生强氧化性羟基自由基( · OH)的绿色氧化反应,选用三聚磷酸钠(STPP)为外加电解质、金刚石为磨粒,比较STPP、NaCl和Na2SO4对芬顿反应中金刚石磨粒分散稳定性的影响,并研究该绿色抛光液在不同pH值下对电芬顿抛光液中金刚石磨粒的抗沉降能力、Zeta电位以及抛光液粒径的影响,并对GaN晶圆的抛光效果进行验证。结果表明:STPP能与芬顿反应抛光液中的Fe2+络合,防止多余的金属阳离子流入扩散层,提高了金刚石磨粒的分散稳定性,且STPP能有效改善芬顿反应抛光液中金刚石磨粒团聚的现象;此外,STPP使抛光液能在偏中性环境中具有较好的分散性,且含有STPP的绿色抛光液可在中性环境下实现对GaN晶圆高效、无损的超精密抛光。Abstract:
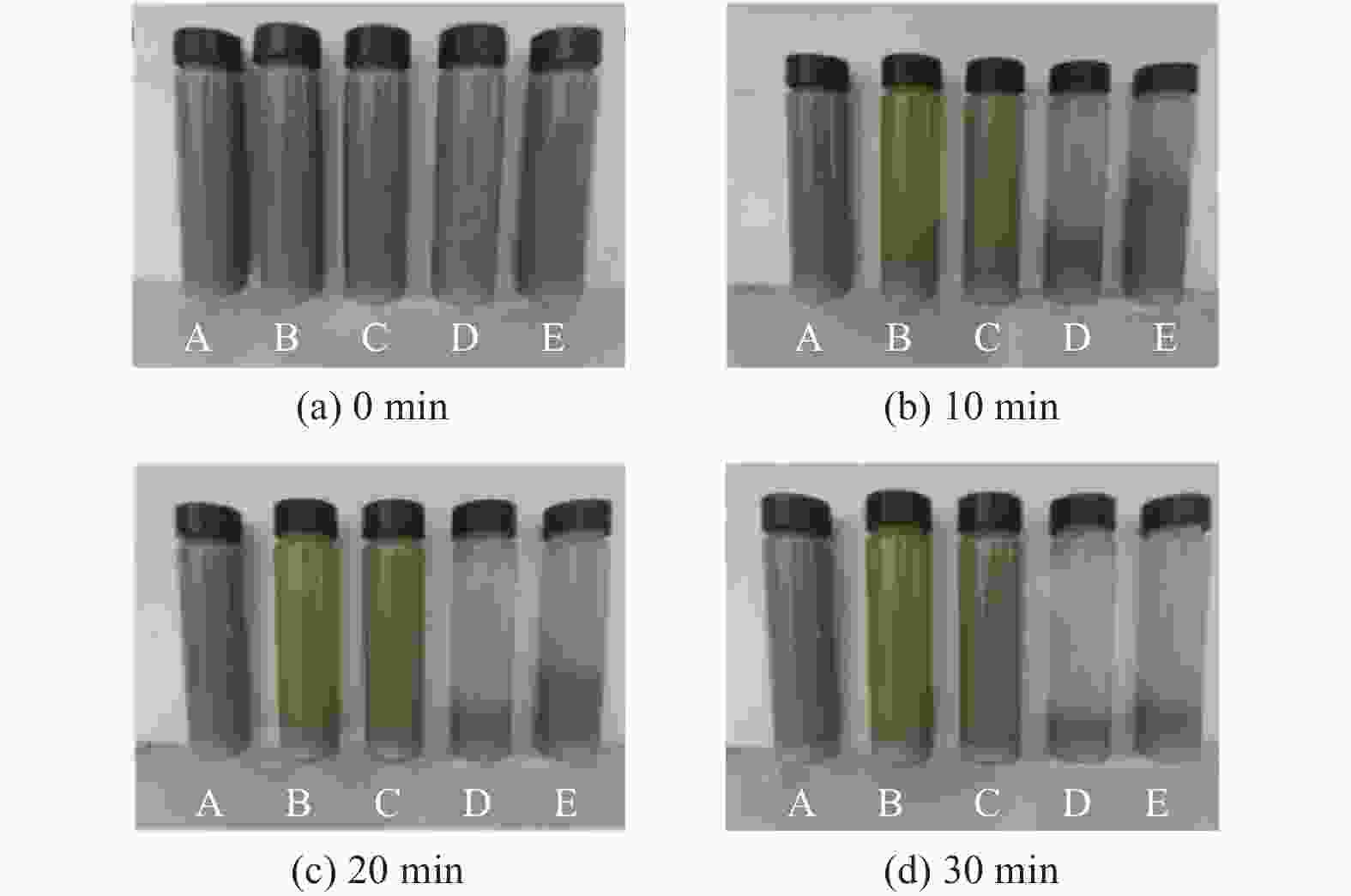
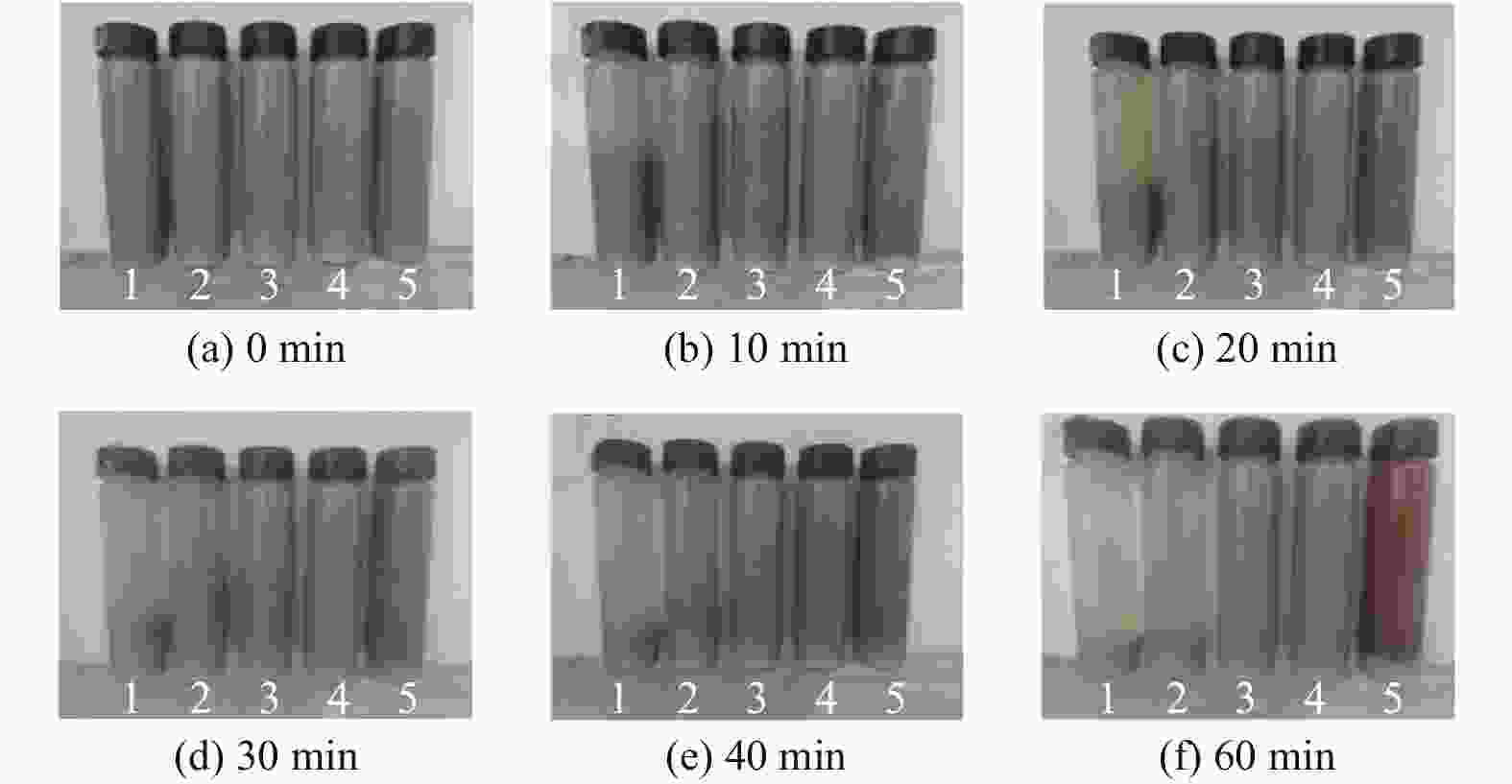
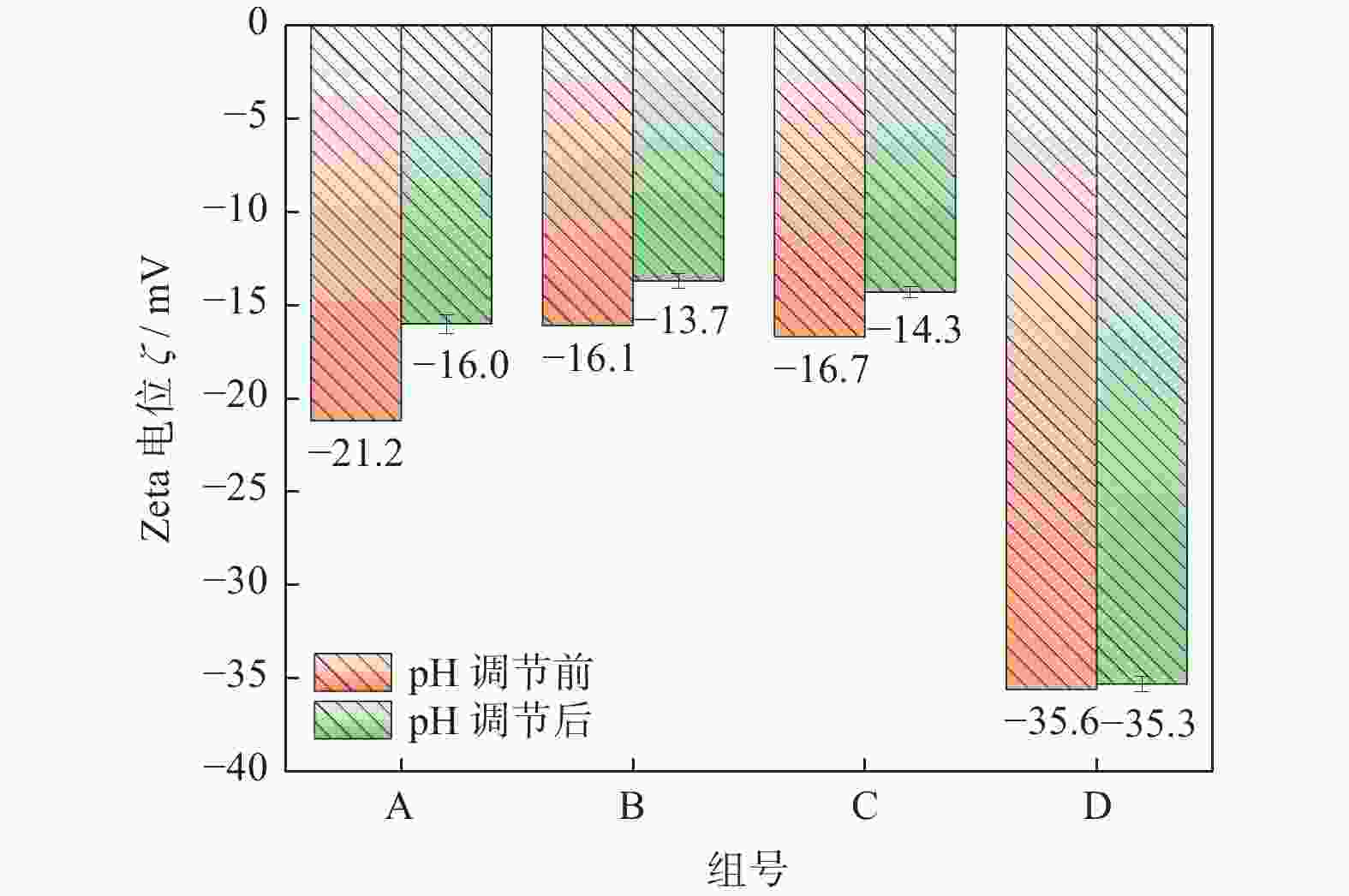
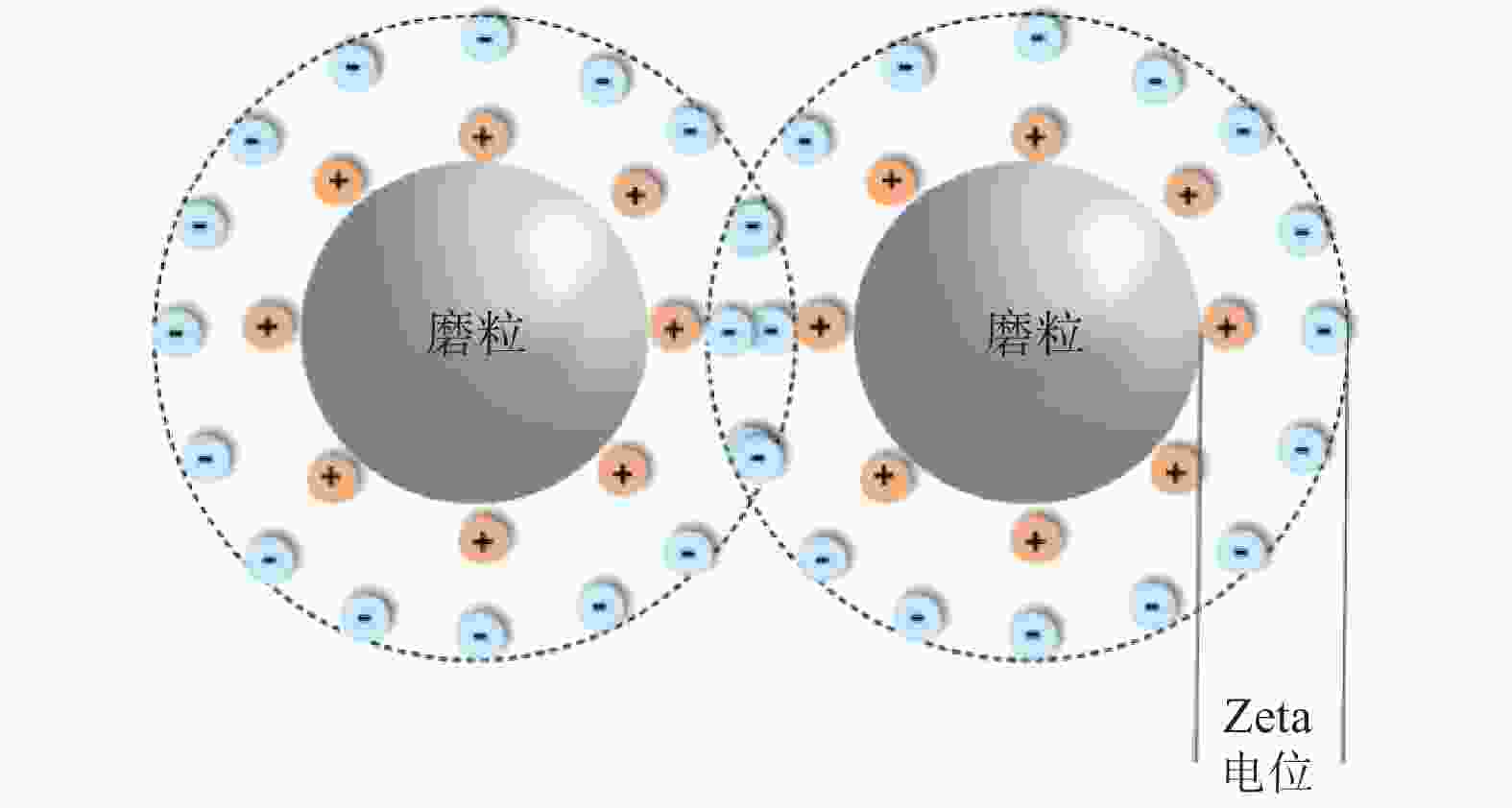
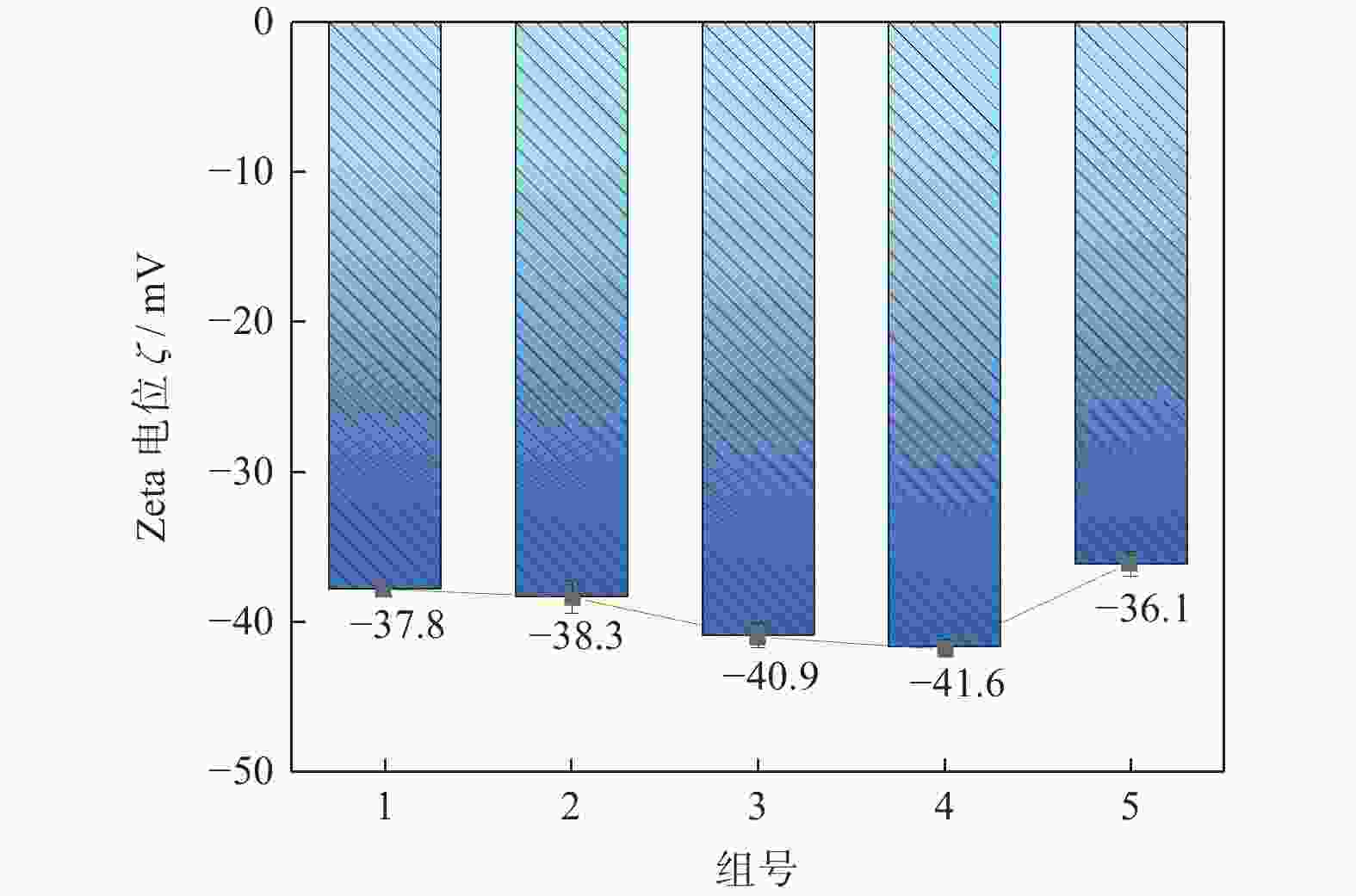
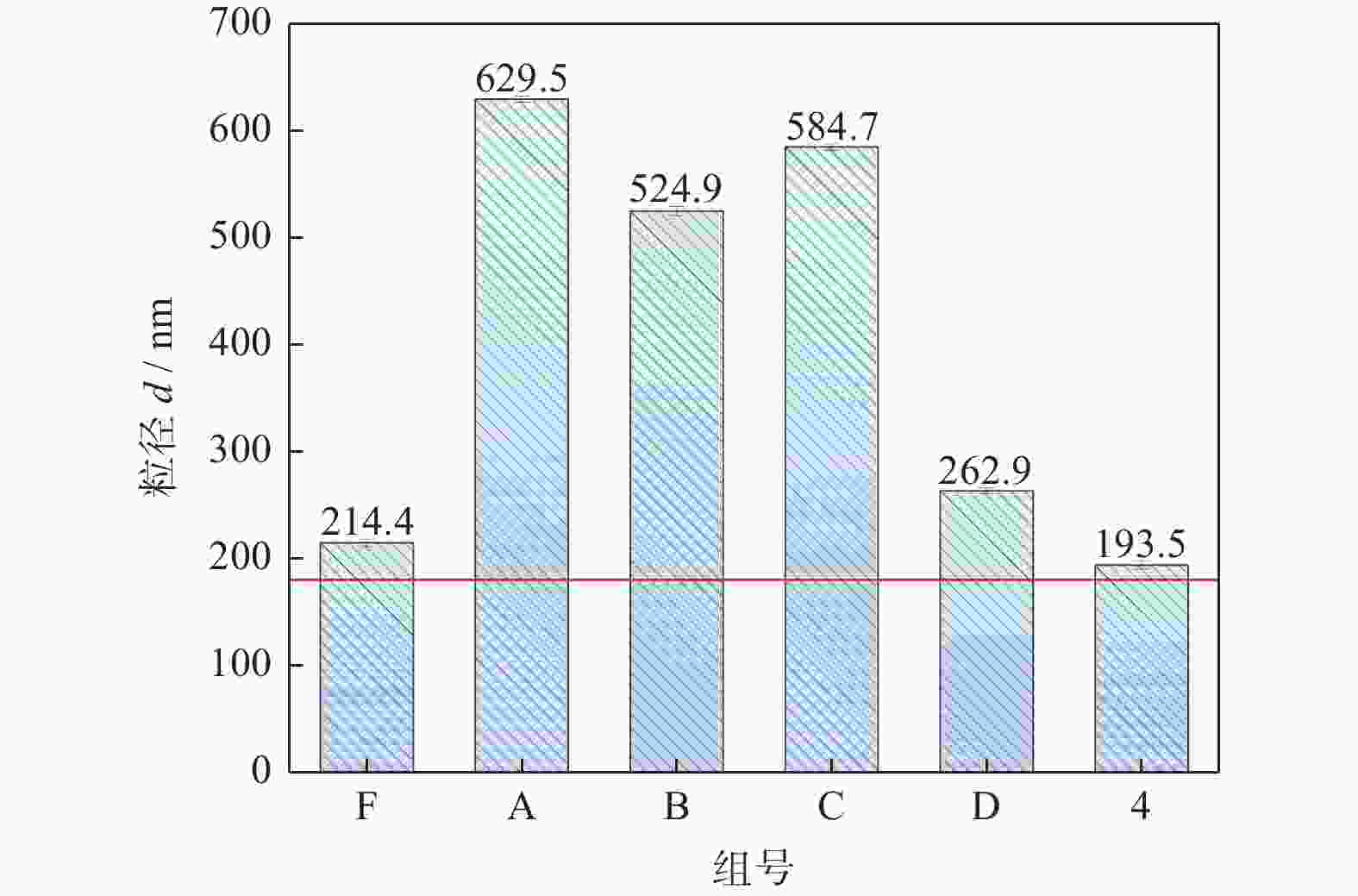
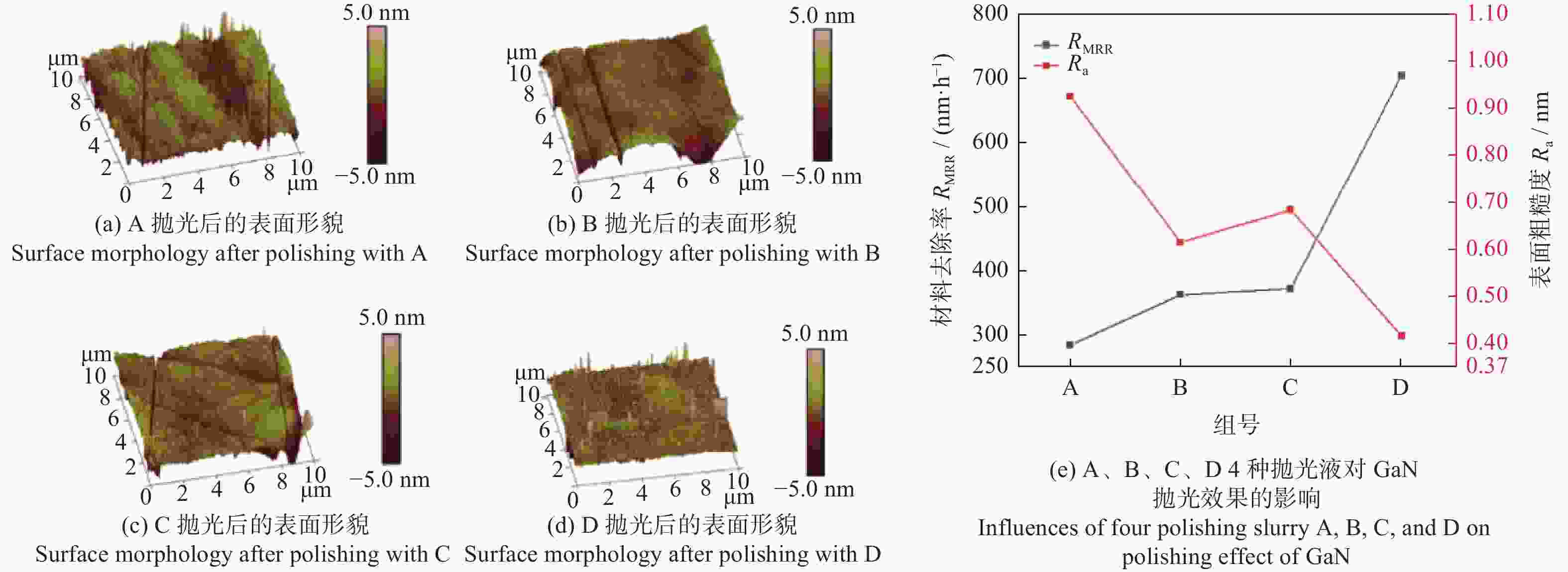
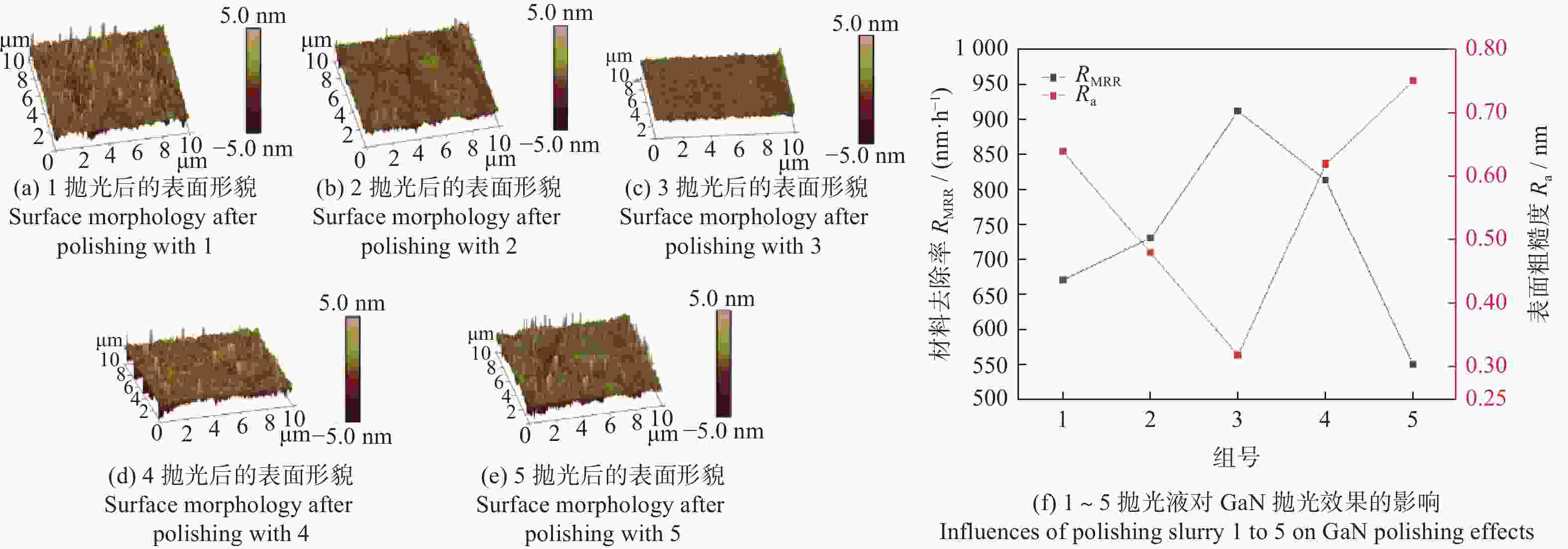
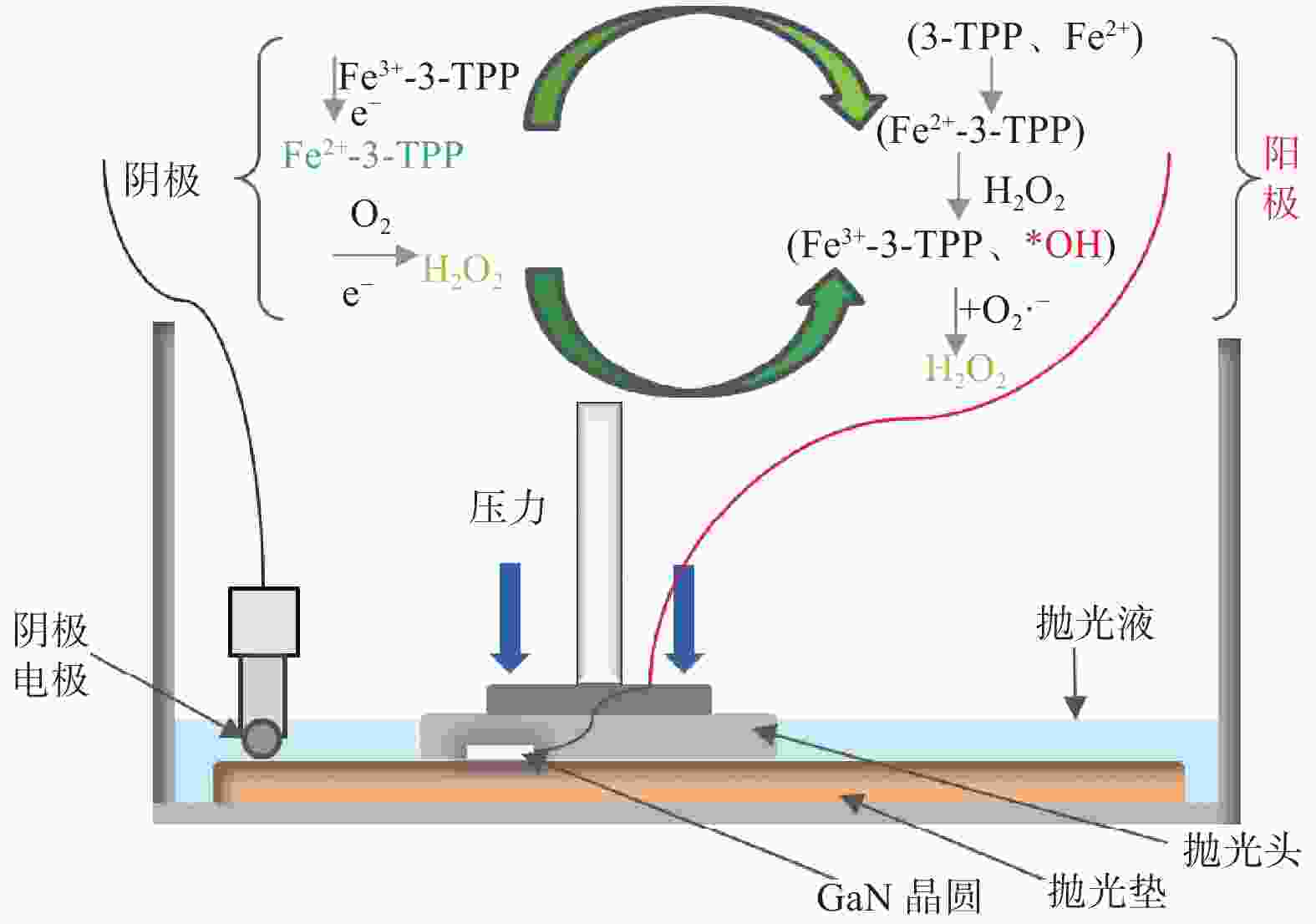
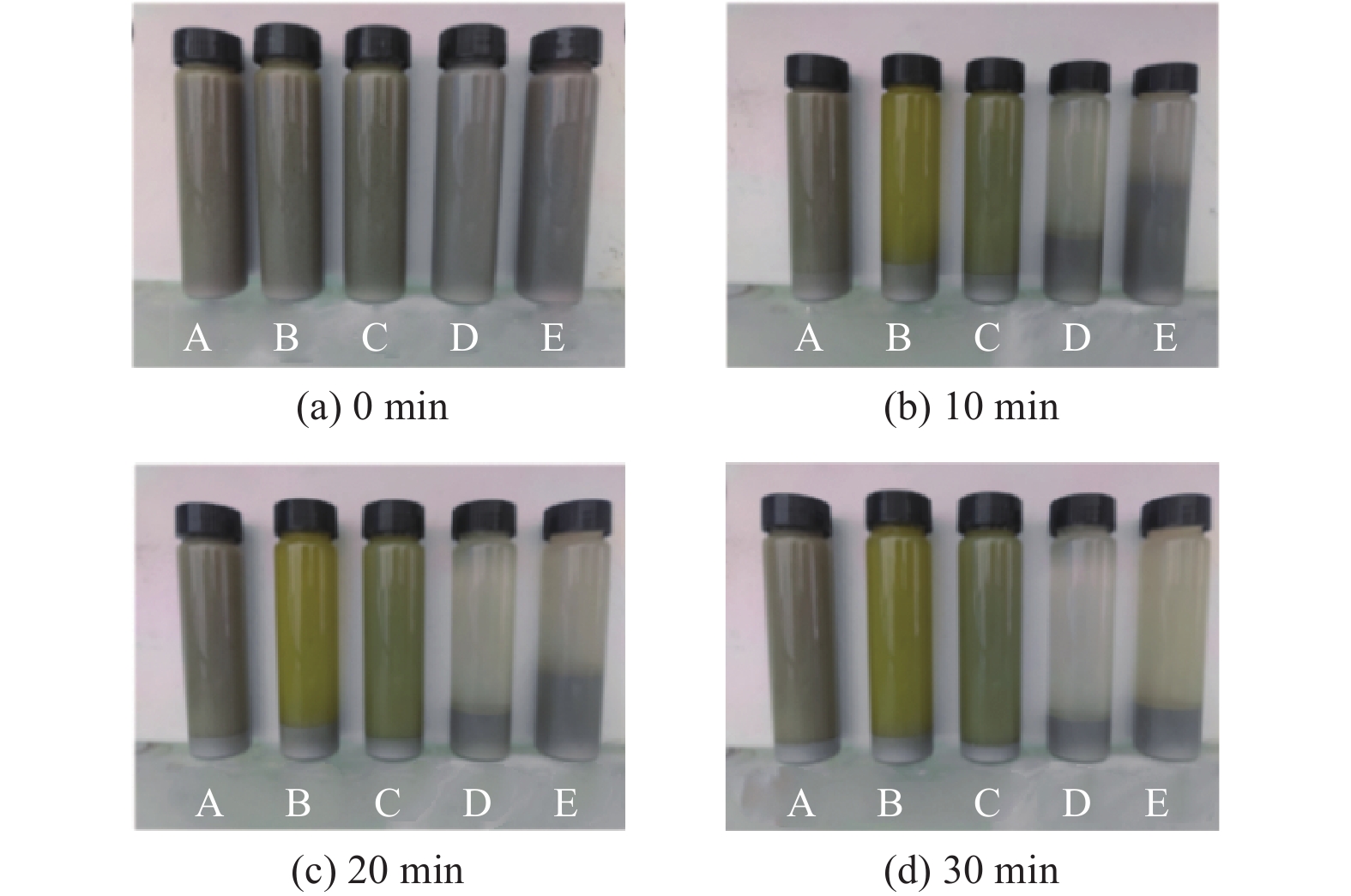
Objectives In the process of GaN ultra-precision polishing, diamond abrasives tend to agglomerate, leading to an increase in the average particle size of abrasives in the polishing slurry, which negatively impacts the surface precision of GaN. The addition of Fe2+ in the Fenton reaction exacerbates this phenomenon. To address this issue, adding electrolytes to the polishing slurry has proven effective in mitigating the agglomeration and sedimentation of abrasives. To compare the effects of STPP, NaCl, and Na2SO4 on the dispersion stability of diamond abrasives during the Fenton reaction, the citric acid and the sodium hydroxide are used as pH regulators to investigate the Fenton reaction of green polishing solution at different pH values. The effects on the anti-settling ability of diamond abrasive particles, the Zeta potential of polishing slurry, and the polishing solution abrasive particle size are studied, and the polishing effect on the GaN wafer is verified. Methods Nano-diamond abrasive particles with an average particle size of 180 nm were added to deionized water, and H2O2 with a mass fraction of 5% and FeSO4·7H2O with a mass of 0.2 g were added as the original reactants of the Fenton reaction. Then, NaCl, Na2SO4 and STPP electrolytes with a mass fraction of 1% were added to prepare three groups of Fenton reaction polishing slurry, which were compared with the original Fenton reaction polishing slurry without any additional electrolyte. After thorough stirring for 5 minutes and ultrasonic dispersion for 10 minutes, citric acid and sodium hydroxide were used to adjust the pH values of all four slurries to 3. At the same time, the polishing slurry with STPP electrolyte at a pH value of 6 was prepared, and the influence of pH value on the anti-settling effect of diamond abrasive particles under STPP electrolyte was preliminarily investigated as the control group. The above five groups of polishing slurries were added to glass bottles for particle settling experiments, and the dispersion stability of diamond abrasive particles in different groups of polishing slurry was observed. The Zetasizer Nano ZS90 nanometer particle size potential analyzer was used to measure the Zeta potential and the particle size of the five groups of polishing slurries before and after the addition of a pH regulator. Finally, the ECMP experiment was carried out to study the effects of green polishing slurries on material removal rate and surface roughness at different pH values. Results (1) When the pH value is 3, the original Fenton polishing slurry and the polishing slurries with NaCl and Na2SO4 electrolytes have weaker anti-settling ability compared to the polishing slurry with STPP added, and the effect of improving the anti-settling ability of diamond abrasives is not obvious. The first three groups of polishing slurries exhibit an obvious delamination phenomenon at the bottom within the first 10 minutes. This indicates that STPP can improve the anti-settling ability of diamond abrasives in Fenton reaction polishing slurry. Furthermore, comparing the polishing slurry samples with STPP added at pH values of 3 and 6, it is found that the anti-settling ability of diamond in the polishing slurry with a pH value of 3 is significantly lower than that in the sample with a pH value of 6, indicating that STPP can improve the anti-settling ability of diamond abrasives in an environment with a pH value of 6. (2) The addition of NaCl and Na2SO4 electrolytes results in a smaller absolute Zeta potential and poorer stability of the polishing slurry, while the addition of STPP greatly increases the dispersion stability of diamond abrasives in the Fenton reaction polishing slurry. At the same time, after adjusting the pH value to 3, the absolute values of the Zeta potential of all polishing slurries slightly decrease compared to before pH adjustment, indicating a decrease in the stability of the polishing slurry. That is, the addition of acidic electrolytes may reduce the stability of the polishing slurry. (3) When the surface of GaN is processed with the polishing slurry containing STPP, there are almost no deep scratches caused by abrasive agglomeration compared with other comparison groups. The surface quality of the processed GaN is the best, with a surface roughness Ra as low as 0.449 nm, and the material removal rate is much higher than that of the control group, reaching up to 705.3 nm/h. Conclusions The Fenton reaction utilizes Fe2+ to react with H2O2 to generate hydroxyl radicals (·OH) with strong oxidizing properties. With the addition of STPP, Fe2+in the solution forms a relatively stable complex Fe2+-STPP with STPP, and which quickly reacts with H2O2 to form Fe3+-STPP and ·OH after the addition of H2O2, effectively avoiding the presence of a large amount of free Fe3+ and the precipitation of Fe(OH)3. Adding STPP to the Fenton reaction polishing slurry with diamond as abrasive particles can broaden the pH range of the Fenton reaction polishing slurry, breaking the limit of pH≤3, and preventing the precipitation of iron flocs in the polishing slurry in a neutral environment. Meanwhile, STPP can enhance the anti-settling ability of diamond abrasives in the Fenton reaction polishing slurry in a neutral environment, and the alkaline pH regulator weakens the anti-settling ability of diamond abrasives in polishing slurry more significantly than the acidic pH regulator. -
表 1 6种抛光液成分
Table 1. Six types of polishing solution components
抛光液组号 外加电解质
种类电解质质量
分数 ω1 / %抛光液pH值 金刚石磨粒质量
分数 ω2 / %FeSO4·7H2O
质量 m / g去离子水 A 无 0 3.0 1 0.2 余量 B NaCl 1 3.0 1 0.2 余量 C Na2SO4 1 3.0 1 0.2 余量 D STPP 1 3.0 1 0.2 余量 E STPP 1 6.0 1 0.2 余量 F 无 0 7.0 1 0 余量 -
[1] 唐林江, 陈滔, 张宝林, 等. GaN基半导体技术的空间应用研究与展望 [J]. 空间电子技术,2018,15(2):60-67. doi: 10.3969/j.issn.1674-7135.2018.02.010TANG Linjiang, CHEN Tao, ZHANG Baolin, et al. Space application research and prospect of GaN-based semiconductor technology [J]. Space Electronics Technology,2018,15(2):60-67. doi: 10.3969/j.issn.1674-7135.2018.02.010 [2] 顾雨萍, 李向江, 吴秀梅. 第三代半导体材料发展前景分析 [J]. 中国集成电路,2023,32(3):22-25. doi: 10.3969/j.issn.1681-5289.2023.03.005GU Yuping, LI Xiangjiang, WU Xiumei. Development prospect of third generation semiconductor materials [J]. China Integrated Circuits,2023,32(3):22-25. doi: 10.3969/j.issn.1681-5289.2023.03.005 [3] ARJUNAN A C, SINGH D, WANG H T, et al. Improved free-standing GaN schottky diode characteristics using chemical mechanical polishing [J]. Applied Surface Science,2008,255(5):3085-3089. doi: 10.1016/j.apsusc.2008.08.096 [4] GAO P L, ZHANG Z Y, WANG D, et al. Research progress of green chemical mechanical polishing slurry [J]. Acta Physica Sinica, 2021, 70(6): 234130440. [5] 杨军, 陈泽鹏, 郭雅茹, 等. 电芬顿降解抗生素废水研究进展 [J]. 当代化工,2023,52(10):2427-2433. doi: 10.13840/j.cnki.cn21-1457/tq.2023.10.037YANG Jun, CHEN Zepeng, GUO Yaru, et al. Research progress on the degradation of antibiotic wastewater by electrofenton [J]. Contemporary Chemical Industry,2023,52(10):2427-2433. doi: 10.13840/j.cnki.cn21-1457/tq.2023.10.037 [6] 马成新, 史小华. 浅谈金刚石工具的现状与发展趋势 [J]. 超硬材料工程,2015,27(5):45-48. doi: 10.3969/j.issn.1673-1433.2015.05.009MA Chengxin, SHI Xiaohua. A brief discussion on the current status and development trends of diamond tools [J]. Journal of Superhard Materials Engineering,2015,27(4):45-28. doi: 10.3969/j.issn.1673-1433.2015.05.009 [7] 董彦辉, 牛风丽, 任泽. 金刚石磁性磨料与SiC磁性磨料的研磨加工性能分析 [J]. 金刚石与磨料磨具工程,2023,43(3):379-385. doi: 10.13394/j.cnki.jgszz.2022.0154DONG Yanhui, NIU Fengli, REN Ze. Analysis of abrasive performance of diamond magnetic abrasives and SiC magnetic abrasives [J]. Diamond and Abrasive Tools Engineering,2023,43(3):379-385. doi: 10.13394/j.cnki.jgszz.2022.0154 [8] DERJAGUIN B, LANDAU L. Theory of the stability of strongly charged lyophobic sols and of the adhesion of strongly charged particles in solutions of electrolytes [J]. Progress in Surface Science,1993,43(1 / 2 / 3 / 4):30-59. doi: 10.1016/0079-6816(93)90013-L [9] ZHU Y, QIU S, DENG F, et al. Enhanced degradation of sulfathiazole by electro-Fenton process using a novel carbon nitride modified electrode [J]. Carbon,2019,145:321-332. doi: 10.1016/j.carbon.2019.01.032 [10] 朱蒙. 氯化钠电解质在电催化氧化体系降解有机污染物的作用研究 [D]. 南昌: 南昌航空大学, 2022.ZHU Meng. Study on the degradation of organic pollutants by sodium chloride electrolyte in electrocatalytic oxidation system [D]. Nanchang: Nanchang Hangkong University, 2022. [11] 程磊. 基于电解质调控的熔石英磁流变抛光机理研究 [D]. 绵阳: 西南科技大学, 2023.CHENG Lei. Research on magnetorheological polishing mechanism of fused quartz based on electrolyte regulation [D]. Mianyang: Southwest University of Science and Technology, 2023. [12] 邓凤霞. 三聚磷酸钠电芬顿体系氧化效能强化及作用机制 [D]. 哈尔滨: 哈尔滨工业大学, 2020.DENG Fengxia. Enhancement of oxidation efficiency of sodium tripolyphosphate electrofenton system and its mechanism [D]. Harbin: Harbin Institute of Technology, 2020. [13] DO T M, BYUN J Y, KIM S H. An electro-Fenton system using magnetite coated metallic foams as cathode for dye degradation [J]. Catalysis Today,2017,295:48-55. doi: 10.1016/j.cattod.2017.05.016 [14] 张碧波, 陈剑, 冉启洋, 等. 柠檬酸改性芬顿对土壤中石油烃的去除效果 [J]. 湖南农业科学,2019(12):38-41. doi: 10.16498/j.cnki.hnnykx.2019.012.009ZHANG Bibo, CHEN Jian, RAN Qiyang, et al. Removal effect of citric acid modified Fenton on petroleum hydrocarbon in soil [J]. Hunan Agricultural Sciences,2019(12):38-41. doi: 10.16498/j.cnki.hnnykx.2019.012.009 -



 下载:
下载:

 邮件订阅
邮件订阅 RSS
RSS
