Effect of Ti3AlC2 content on properties of polycrystalline diamond
-
摘要: 结合剂的类型和含量对聚晶金刚石的性能有很大的影响。以5 μm金刚石为原材料,选用MAX相中的Ti3AlC2为结合剂,在5.5 GPa、
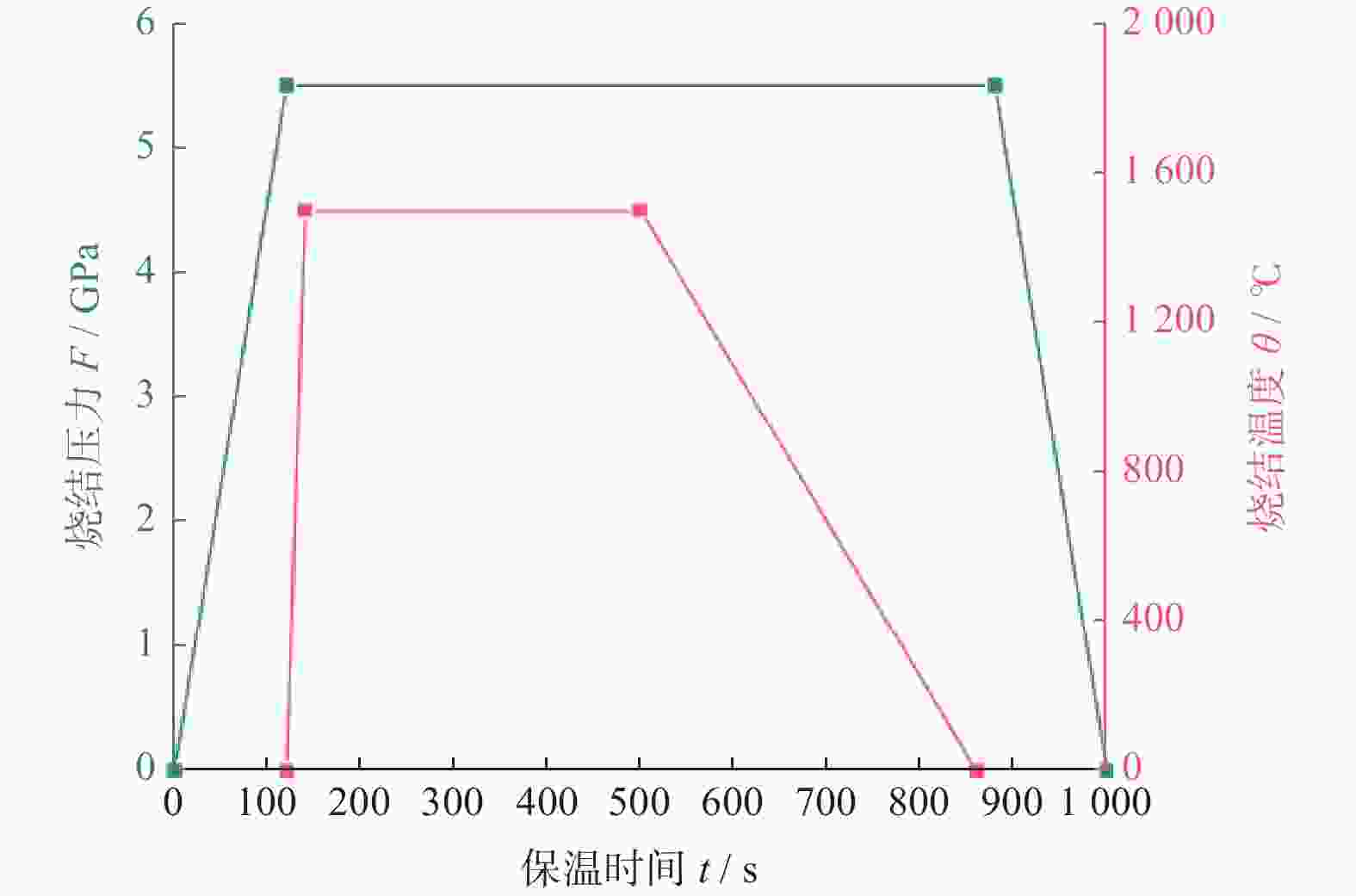
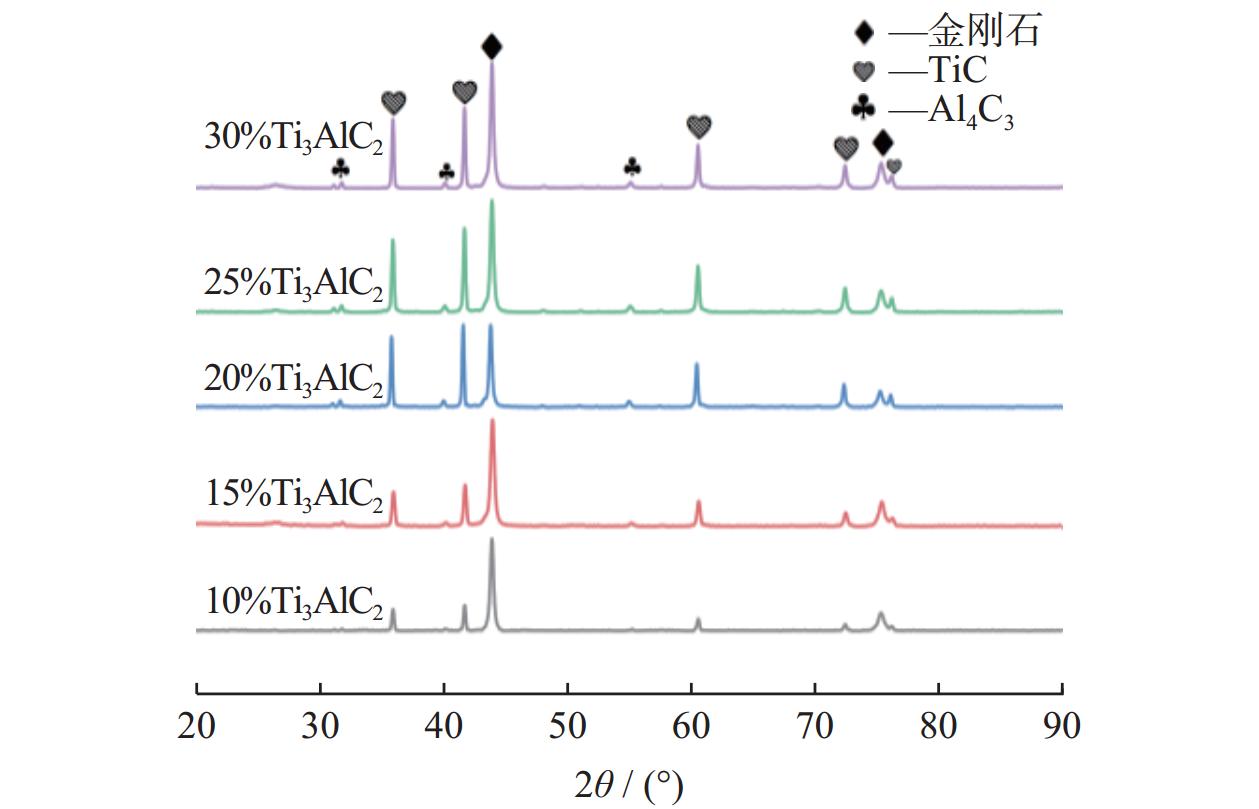
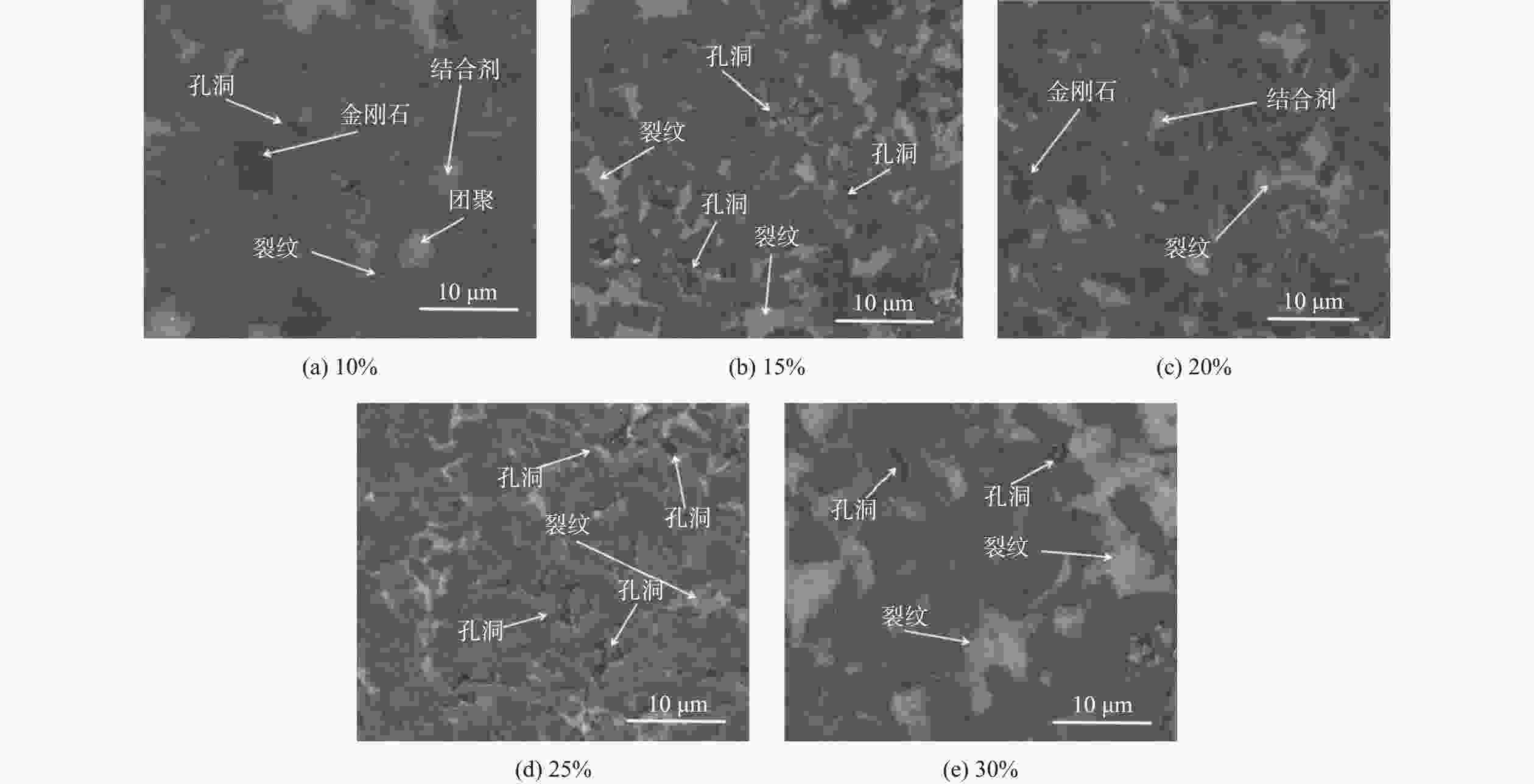
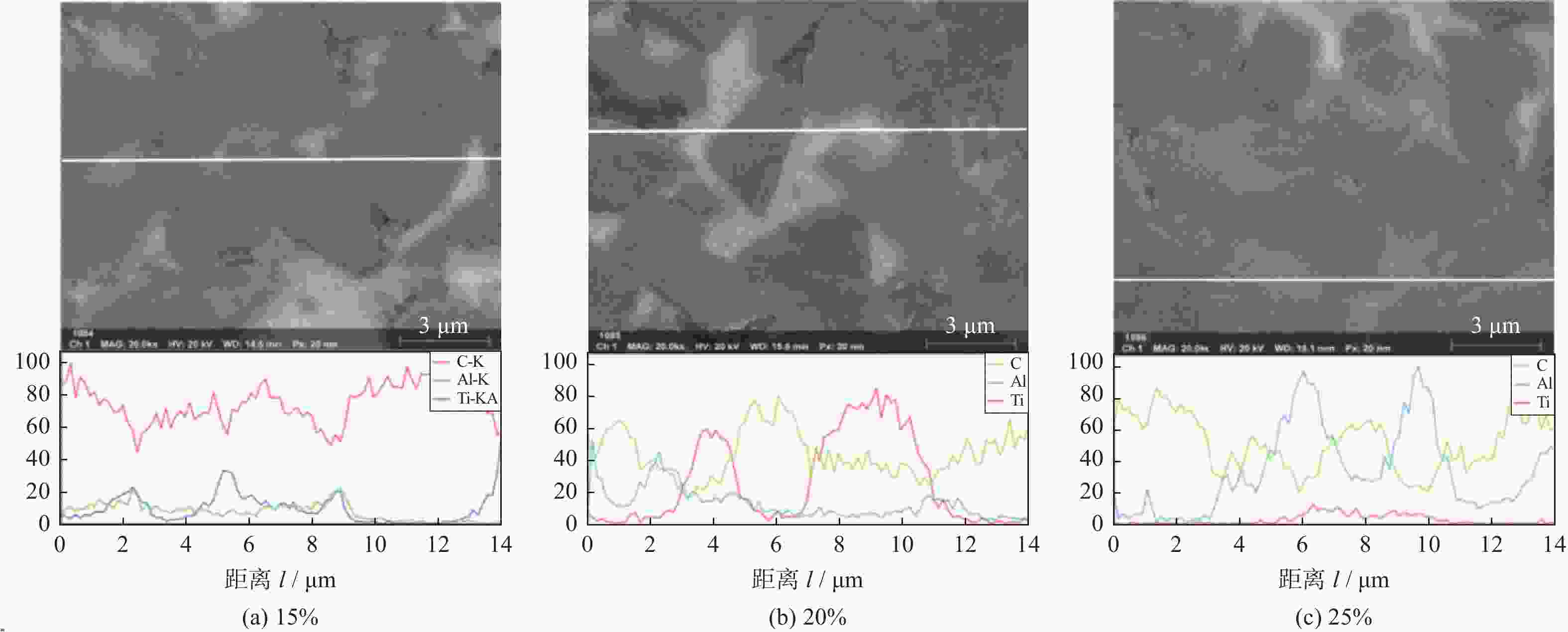
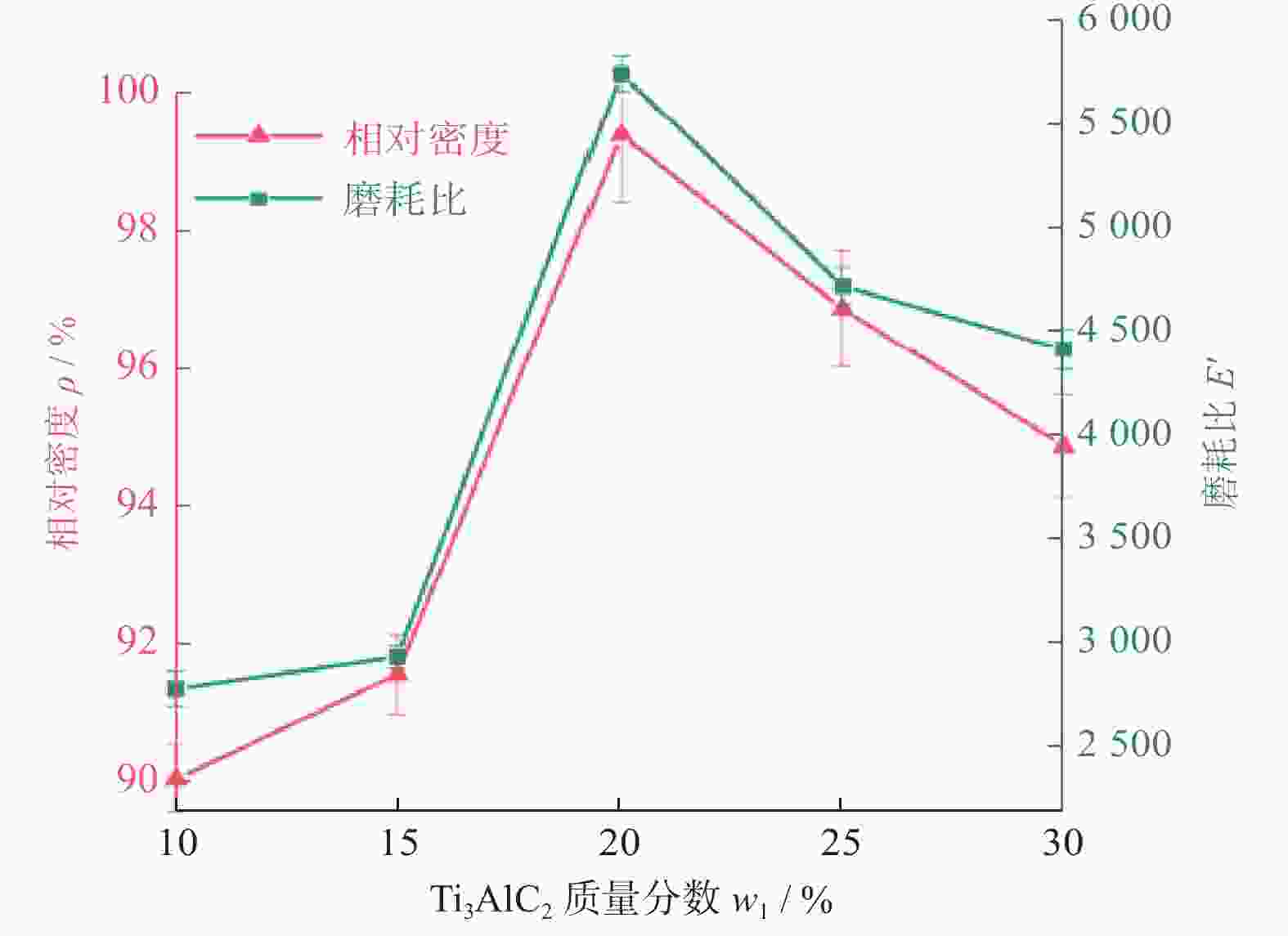
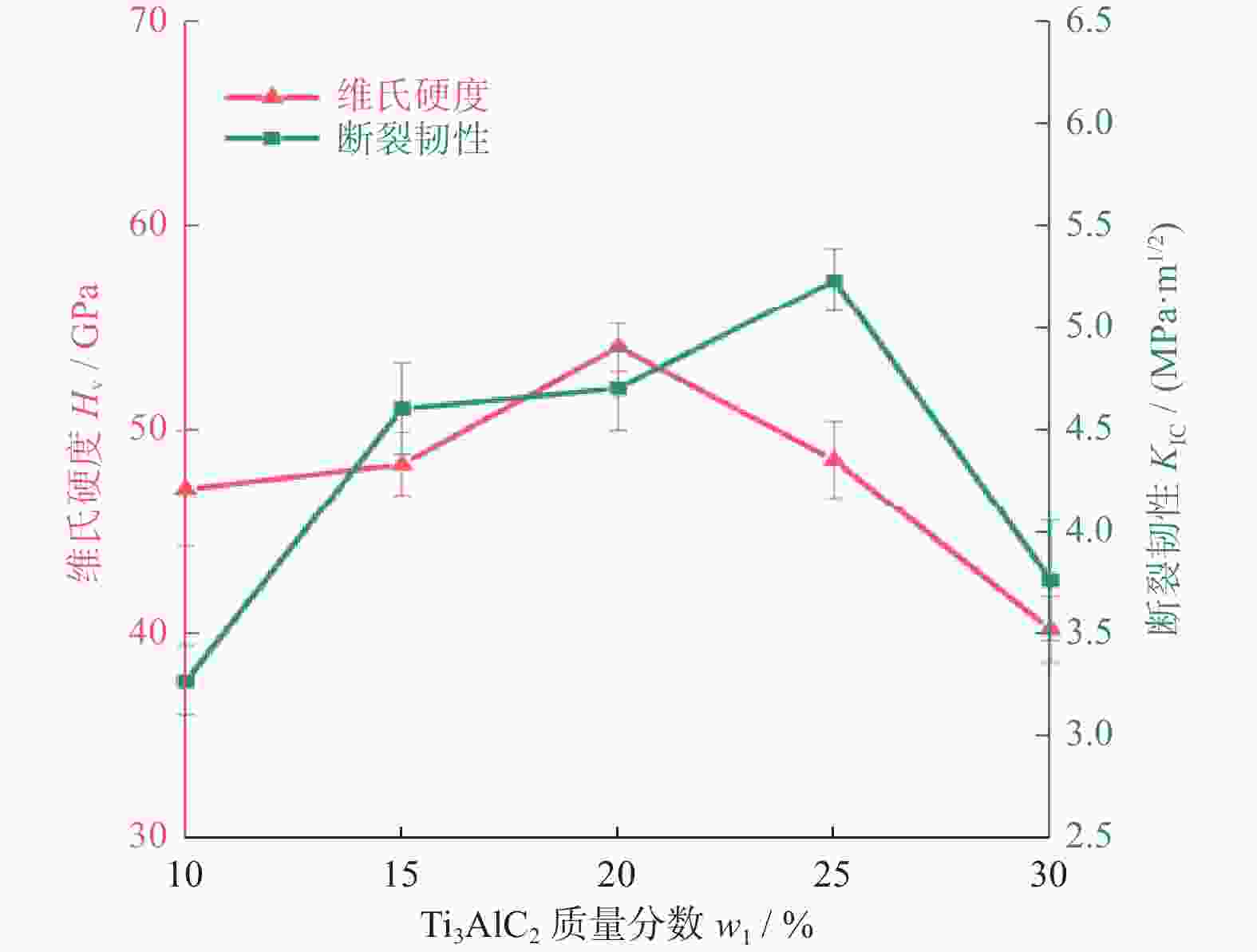
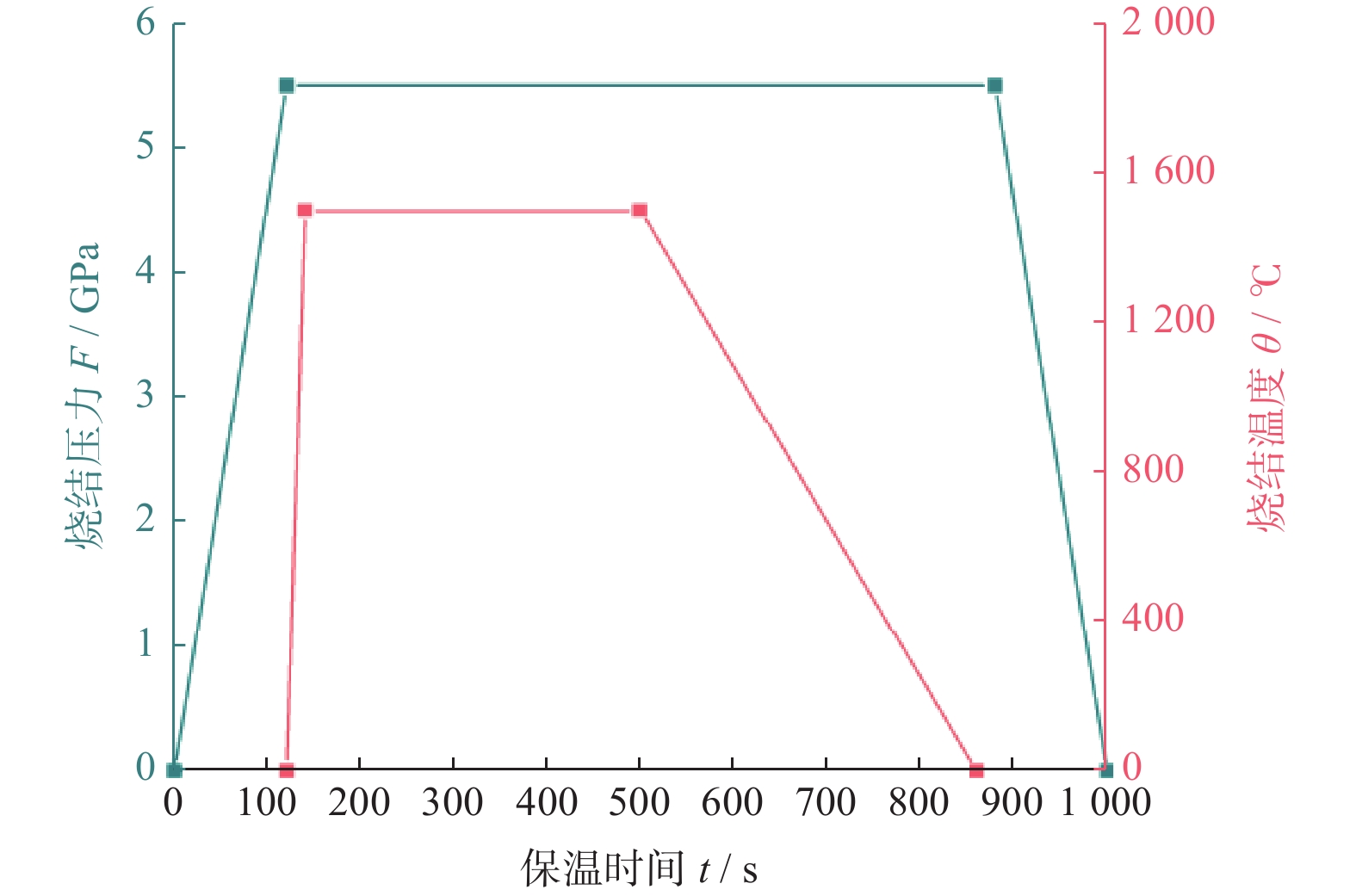
1500 ℃下制备不同Ti3AlC2含量的聚晶金刚石,分析了Ti3AlC2的含量对聚晶金刚石的物相、显微结构及力学性能的影响。结果表明:Ti3AlC2在高温高压下会完全分解形成TiC和Al-Ti合金,并与金刚石反应生成Al4C3和TiC等物相,且TiC和Al4C3均匀分布在金刚石颗粒间并与金刚石紧密黏结在一起,从而提升聚晶金刚石的力学性能。当Ti3AlC2质量分数为20%时,聚晶金刚石的相对密度、维氏硬度和磨耗比均达到最大值,分别为99.3%、54.0 GPa和5733.3 ;当Ti3AlC2质量分数为25%时,聚晶金刚石的断裂韧性达到最大值5.23 MPa·m1/2。Abstract: Objectives: In superhard materials, polycrystalline diamond (PCD) has become a hot topic in scientific research because it inherits the advantages such as high hardness, high wear resistance, and high thermal conductivity from diamond. The type and content of the binder have a great influence on the properties of polycrystalline diamond. Methods: The microstructure of polycrystalline diamond samples is analyzed by FEI INSPECT F50 scanning electron microscope (SEM) of FEI Company in the United States, and the bonding state between diamond and binder is observed. The distribution of each element in the polycrystalline diamond sintered body is tested and analyzed by an energy dispersive spectrometer attached to the scanning electron microscope. An A8 ADVANCE X-ray diffractometer (XRD, λ = 0.154 06 nm, Germany, scanning speed: 10°/ min, scanning range: 20°~90°) is used to analyze the phase of polycrystalline diamond samples and raw materials to determine their phase composition. The sample density is measured by Archimedes' principle. The Vickers hardness of the sample is measured using a FM-ARS900 microhardness tester (load: 9.81 N, holding time: 10s). The fracture toughness is calculated using the fracture toughness formula according to the crack length, the hardness, and the elastic modulus of the material. Results: It can be concluded from XRD analysis that PCD is mainly composed of diamond, TiC, and Al4C3, and that the Ti3AlC2 phase is not detected in any sample. Therefore, Ti3AlC2 is considered to have been completely decomposed. The Ti-C bond in Ti3AlC2 is a strong covalent bond, while the Ti-Al bond is a weak metallic bond. In addition, Ti3AlC2 is a metastable phase, while TiC is stable at high pressure and high temperature. Therefore, Ti3AlC2 is decomposed into TiC and Al-Ti alloy, and Al-Ti reacts with diamond to form TiC and Al4C3. The existence of TiC and Al4C3 diffraction peaks also confirms this finding. From the SEM analysis, it can be concluded that when the binder content is 10% and 15%, there are a small number of holes and cracks on the surface of PCD; when the binder content is 20%, the diamond is tightly wrapped by the binder, and there are no obvious holes between the particles. The crystal form is complete without any fragmentation, and the diamond is arranged closely and distributed evenly, with better combination and compactness, indicating that the synthesis process of the PCD sintered body is well controlled. When the binder content is 25% and 30%, the excess binder appears to aggregate and cause dispersed diamonds and more holes. Moreover, no Ti3AlC2 grains with a layered structure were found under these conditions, which was consistent with the results of XRD analysis. Under high temperature and high pressure, the increase in Ti3AlC2 content will decompose more Al-Ti alloy into the liquid phase, which enhances the flow and uniform distribution of diamond and the generated hard phases in the system. At the same time, the product of Ti3AlC2 decomposition reacts with diamond under high temperature and high pressure to form TiC and Al4C3 with strong covalent bonds, which improves the bonding state between diamond particles, thus improving the comprehensive mechanical properties of PCD. The relative density shows a trend of increasing first and then decreasing. When the amount of binder is too much, the bonding performance between diamond and binder becomes worse, and the wear ratio decreases. In the electron microscope image, it is observed that the aggregation and porosity of the binder appear in the PCD composites, which explains the deterioration of mechanical properties. With the increase of Ti3AlC2 content, the Vickers hardness and fracture toughness of PCD increase first and then decrease. When the mass fraction of Ti3AlC2 is 20%, the hardness of PCD reaches the maximum value of 54.0 GPa. The fracture toughness of PCD reaches the maximum value of 5.23 MPa·m1/2 when the mass fraction of Ti3AlC2 is 25%. When the mass fraction of Ti3AlC2 is more than 20%, the high content of binder in the sintered body will lead to a decrease in the relative density of PCD, resulting in a decrease in the Vickers hardness of the sample. Conclusions: Polycrystalline diamond is prepared at 5.5 GPa,1500 °C, and 6 minutes by using diamond with a particle size of 5 μm as raw material and Ti3AlC2 as the binder. The effect of Ti3AlC2 content on the structure and properties of PCD is studied. Ti3AlC2 completely decomposes and reacts with diamond under high temperature and high pressure. Diamond, TiC, and Al4C3 are the main components in the sintered body. The binder reacts with diamond to form TiC and Al4C3. Through the combination of TiC and Al4C3 as intermediates, the diamond particles are firmly bonded together to form a dense microstructure. An appropriate amount of binder makes the diamond and the bonding material evenly distributed, and the PCD sintered body becomes denser. When the mass fraction of Ti3AlC2 is 20%, the relative density, Vickers hardness, and wear ratio of PCD reach maximum values of 99.3%, 54.0 GPa and 5 733.3, respectively. When the mass fraction of Ti3AlC2 is 25%, the fracture toughness of PCD reaches the maximum value of 5.23 MPa·m1/2. When the Ti3AlC2 content reaches 25% and 30%, the density, hardness, and wear ratio of the PCD samples decrease due to the excessive aggregation and pores of the binder and diamond in the sintered body. -
表 1 实验配方
Table 1. Experimental formula
编号 Ti3AlC2质量分数 w1 / % 金刚石质量分数 w2 / % a 10 90 b 15 85 c 20 80 d 25 75 e 30 70 -
[1] 郑艳彬, 姜志刚, 朱品文. 聚晶金刚石的高温高压制备及其性能研究进展 [J]. 材料导报,2016,30(23):81-86 doi: 10.11896/j.issn.1005-023X.2016.23.012ZHENG Yanbin, JIANG Zhigang, ZHU Pinwen. Research progress on preparation and properties of polycrystalline diamond at high temperature and high pressure [J]. Materials Reports,2016,30(23):81-86 doi: 10.11896/j.issn.1005-023X.2016.23.012 [2] 代晓南, 白玲, 栗正新, 等. 聚晶金刚石高温高压合成工艺研究进展 [J]. 超硬材料工程,2021,33(5):41-45. doi: 10.3969/j.issn.1673-1433.2021.05.010DAI Xiaonan, BAI Ling, LI Zhengxin, et al. Research progress of high temperature and high pressure synthesis process of polycrystalline diamond [J]. Superhard Material Engineering,2021,33(5):41-45. doi: 10.3969/j.issn.1673-1433.2021.05.010 [3] IRIFUNE T, ISOBE F, SHINMEI T. A novel large-volume Kawai-type apparatus and its application to the synthesis of sintered bodies of nano-polycrystalline diamond [J]. Physics of the Earth and Planetary Interiors,2014,228:255-261. doi: 10.1016/j.pepi.2013.09.007 [4] XU C, HE D, WANG H, et al. Nano-polycrystalline diamond formation under ultra-high pressure [J]. International Journal of Refractory Metals and Hard Materials,2013,36:232-237. doi: 10.1016/j.ijrmhm.2012.09.004 [5] WANG H, HE D, XU C, et al. Nanostructured diamond-TiC composites with high fracture toughness[J]. Journal of Applied Physics, 2013: 113-117. [6] HUANG Q, YU D, XU B, et al. Nanotwinned diamond with unprecedented hardness and stability [J]. Nature,2014,510(7504):250-253. doi: 10.1038/nature13381 [7] 崔喜伟, 秦越, 毛荣琪, 等. 合成聚晶金刚石过程的颗粒冷压破碎 [J]. 金刚石与磨料磨具工程,2023,43(4):440-446. doi: 10.13394/j.cnki.jgszz.2022.0178CUI Xiwei, QIN Yue, MAO Rongqi, et al. Cold crushing of particles in the process of synthesizing polycrystalline diamond [J]. Diamond & Abrasives Engineering,2023,43(4):440-446. doi: 10.13394/j.cnki.jgszz.2022.0178 [8] 陈卫民, 李磊, 刘杰, 等. 钴的脱除工艺对聚晶金刚石复合片性能的影响 [J]. 硬质合金,2023,40(4):271-278. doi: 10.3969/j.issn.1003-7292.2023.04.004CHEN Weimin, LI Lei, LIU Jie, et al. Effect of cobalt removal process on properties of polycrystalline diamond compacts [J]. Cemented Carbide,2023,40(4):271-278. doi: 10.3969/j.issn.1003-7292.2023.04.004 [9] JAWORSKA L, SZUTKOWSKA M, KLIMCZYK P, et al. Oxidation, graphitization and thermal resistance of PCD materials with the various bonding phases of up to 800 ℃ [J]. International Journal of Refractory Metals and Hard Materials,2014,45:109-116. doi: 10.1016/j.ijrmhm.2014.04.003 [10] KÜMMEL J, BRAUN D, GIBMEIER J, et al. Study on micro texturing of uncoated cemented carbide cutting tools for wear improvement and built-up edge stabilisation [J]. Journal of Materials Processing Technology,2015,215:62-70. doi: 10.1016/j.jmatprotec.2014.07.032 [11] 高丽娜, 陈文革, 李树丰. Ti3AlC2陶瓷粉末的研究现状及进展 [J]. 材料导报,2022,36(20):170-180. doi: 10.11896/cldb.20090196GAO Lina, CHEN Wenge, LI Shufeng. Research status and progress of Ti3AlC2 ceramic powders [J]. Materials Reports,2022,36(20):170-180. doi: 10.11896/cldb.20090196 [12] 梁宝岩, 张旺玺, 王艳芝, 等. 微波烧结制备MAX相–金刚石复合材料 [J]. 金刚石与磨料磨具工程,2016,36(1):25-30. doi: 10.13394/j.cnki.jgszz.2016.1.0006LIANG Baoyan, ZHANG Wangxi, WANG Yanzhi, et al. MAX phase-diamond composites were prepared by microwave sintering [J]. Diamond & Abrasives Engineering,2016,36(1):25-30. doi: 10.13394/j.cnki.jgszz.2016.1.0006 [13] PRIKHNA T A, STAROSTINA A V, LIZKENDORF D, et al. Studies of the oxidation stability, mechanical characteristics of materials based on max phases of the Ti-Al-(C, N) systems, and of the possibility of their use as tool bonds and materials for polishing [J]. Journal of Superhard Materials,2014,36:9-17. doi: 10.3103/S106345761401002X [14] 朱春城, 钱旭坤, 赫晓东, 等. 燃烧合成Ti3AlC2及其热稳定性 [J]. 稀有金属材料与工程,2009,38(S2):86-89.ZHU Chuncheng, QIAN Xukun, HE Xiaodong, et al. Combustion synthesis of Ti3AlC2 and its thermal stability [J]. Rare Metal Materials and Engineering,2009,38(S2):86-89. [15] 李子扬, 寇自力, 安佩, 等. Ti3AlC2在静高压下的热稳定性 [J]. 材料研究学报,2010,24(4):368-372.LI Ziyang, KOU Zili, AN Pei, et al. Thermal stability of Ti3AlC2 under static high pressure [J]. Chinese Journal of Materials Research,2010,24(4):368-372. [16] HOU M, GAO J, YANG H, et al. Synthesis of diamond composites via microwave sintering and the improvement of mechanical properties induced by in-situ decomposition of Ti3AlC2 [J]. Ceramics International,2021,47(9):13199-13206. doi: 10.1016/j.ceramint.2021.01.185 [17] 钱旭坤. 层状Ti3AlC2的燃烧合成及其性能研究 [D]. 哈尔滨: 哈尔滨工业大学, 2010.QIAN Xukun. Combustion synthesis and properties of layered Ti3AlC2 [D]. Harbin: Harbin Institute of Technology, 2010. [18] YANG L, GONG J, YUE Z, et al. Preparation and characterization of cBN-based composites from cBN-Ti3AlC2 mixtures [J]. Diamond and Related Materials,2016,66:183-187. doi: 10.1016/j.diamond.2016.05.003 [19] 吕晓鑫. Ti基聚晶金刚石复合材料的制备与性能研究 [D]. 天津: 天津大学, 2019.LV Xiaoxin. Preparation and properties of Ti-based polycrystalline diamond composite materials [D]. Tianjing: Tianjin university, 2019. [20] 中华人民共和国工业和信息化部. 聚晶金刚石磨耗比测定方法:JB/T 3235-2013 [S]. 2013-04-25.Ministry of Industry and Information Technology of the People's Republic of China. Testing method for abrasion ratio of polycrystalline diamond: JB/T 3235-2013 [S]. 2013-04-25. [21] 蔡明. Ti3AlC2基复合材料的制备及性能研究 [D]. 沈阳: 沈阳理工大学, 2020.CAI Ming. Preparation and properties of Ti3AlC2 matrix composites [D]. Shenyang: Shenyang Ligong University, 2020. [22] 伊加成. 双连续相Ti3AlC2/2024Al复合材料的制备及其高温力学性能 [D]. 北京: 北京交通大学, 2022.YI Jiacheng. Preparation and high temperature mechanical properties of co-continuous phase Ti3AlC2/2024Al composites [D]. Beijing: Beijing Jiaotong University, 2022. -




 下载:
下载:



 邮件订阅
邮件订阅 RSS
RSS
