Preparation of homogeneous sub-micron cerium oxide polishing powder
-
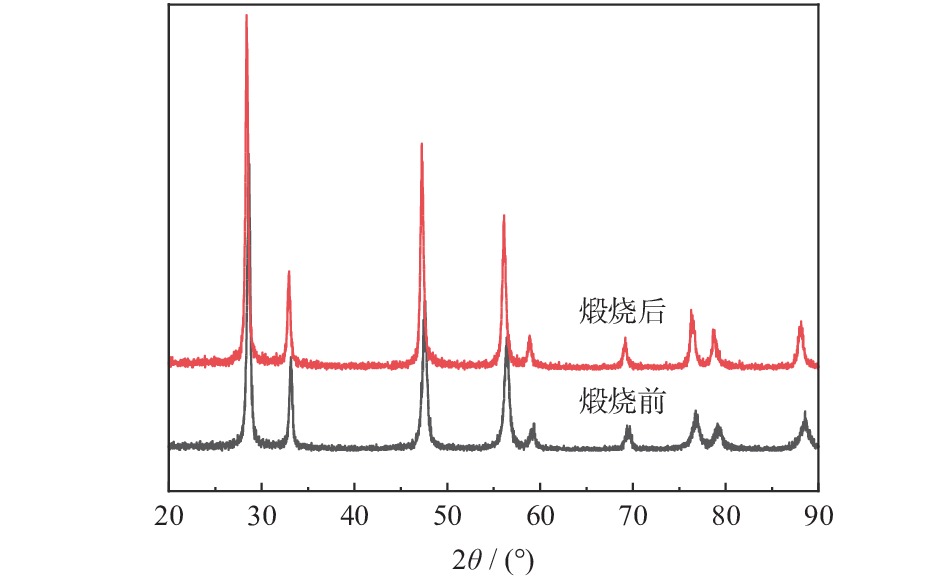
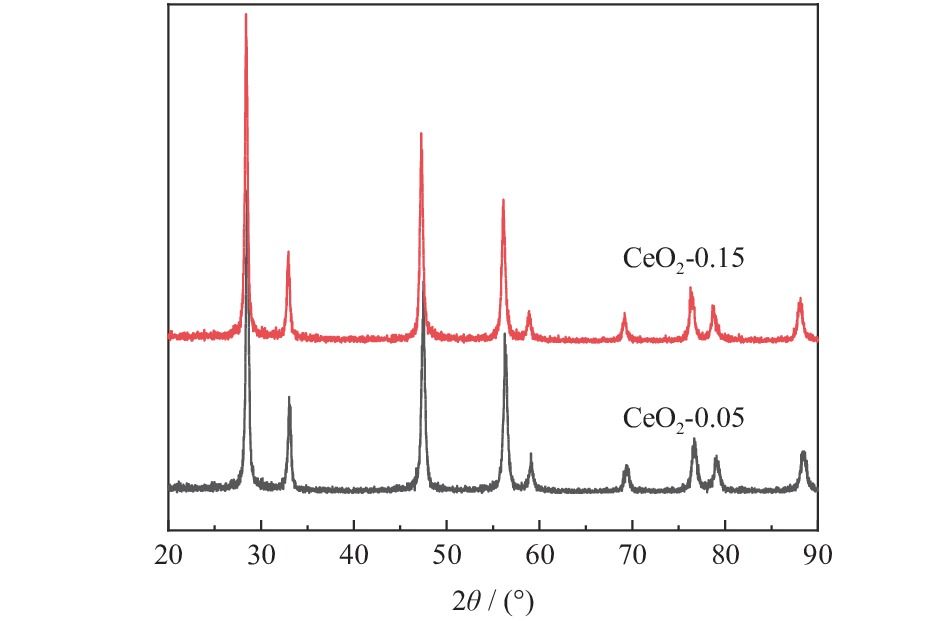
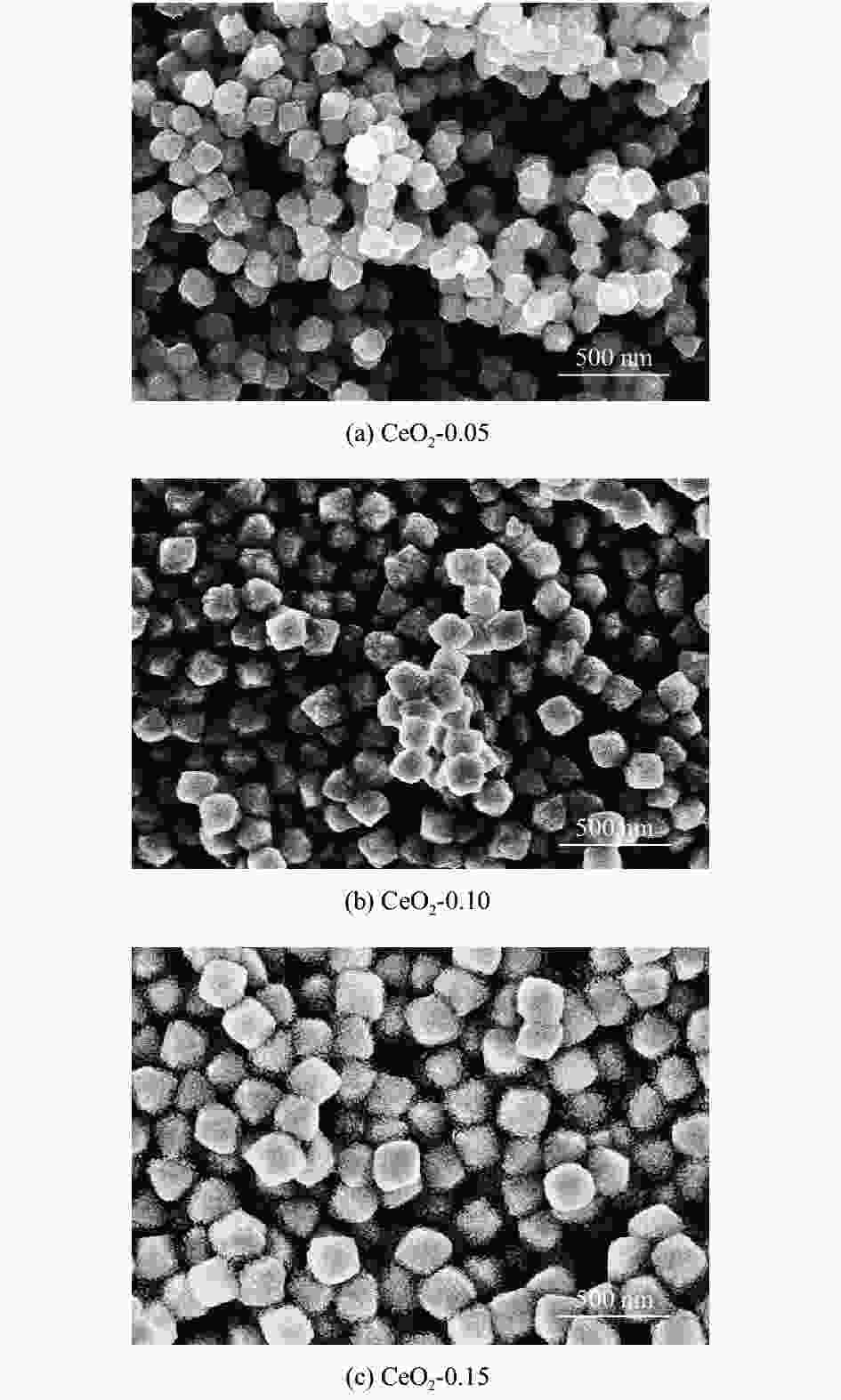
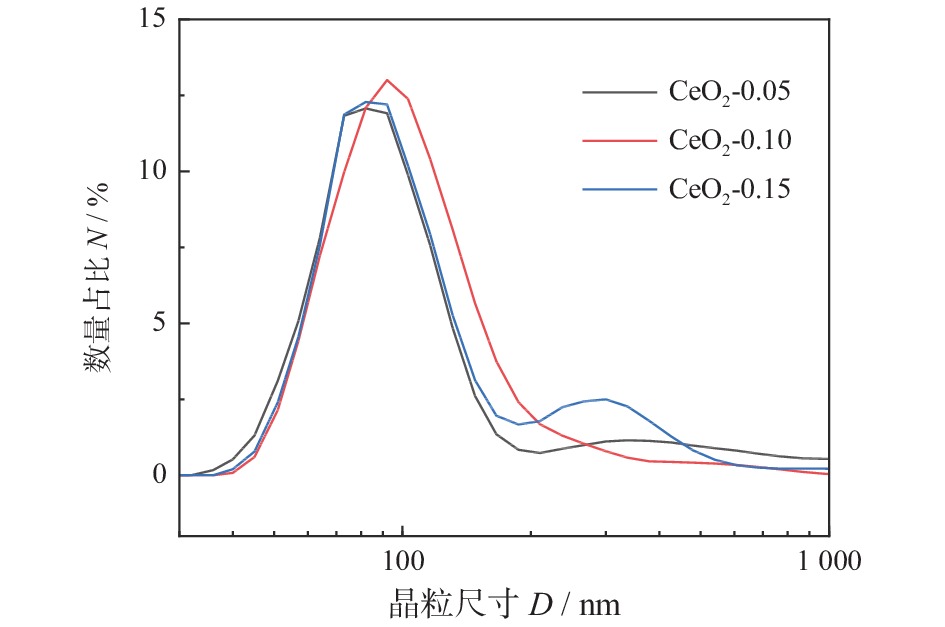
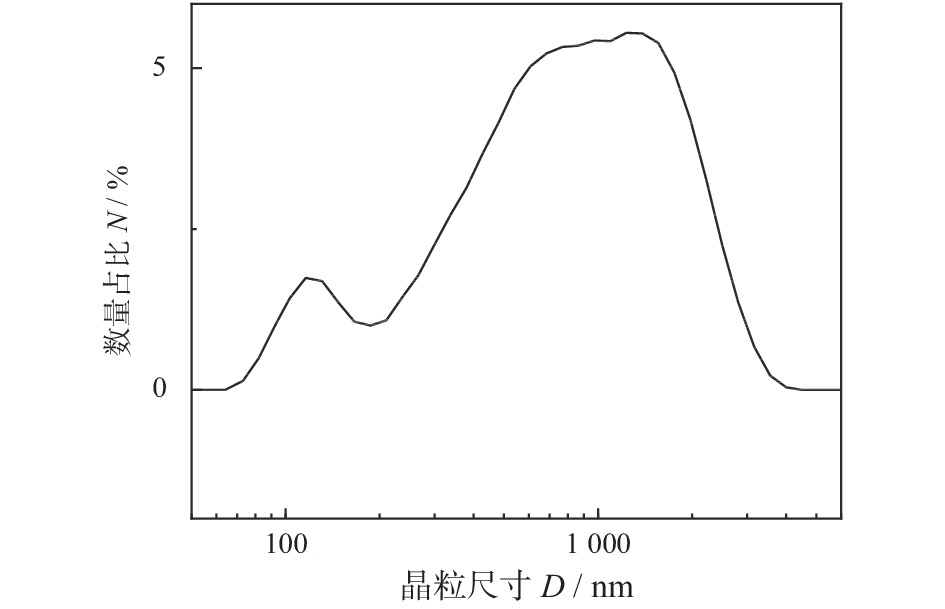
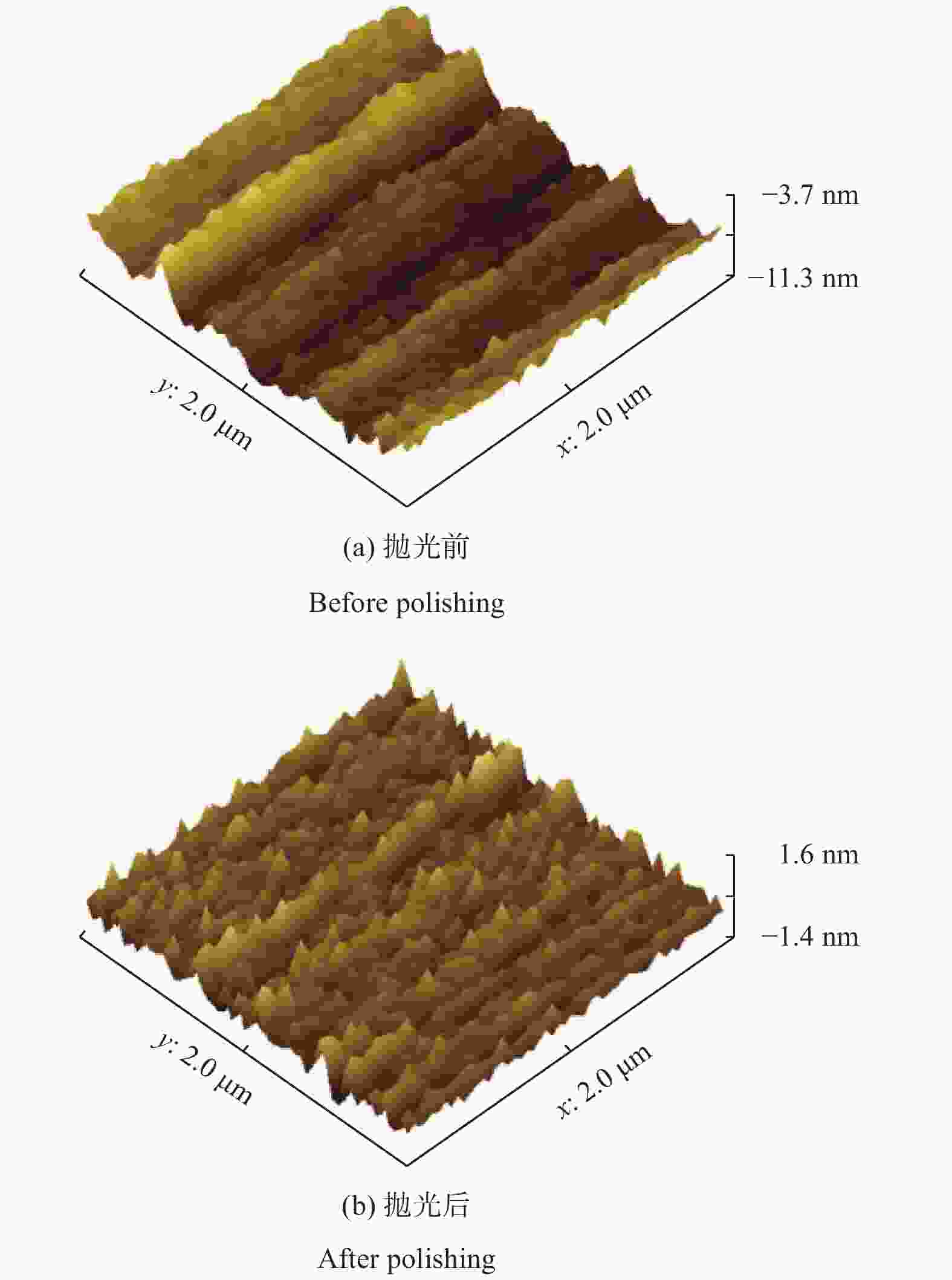
摘要: 为得到均一的亚微米级二氧化铈(CeO2)抛光粉,以六水合硝酸铈(Ce(NO3)3·6H2O)为原料,以醇-水混合溶液为溶剂,采用溶剂热法合成CeO2。改变Ce3+浓度和醇-水体积比,利用X射线衍射仪(XRD)、激光粒度分布仪、扫描电子显微镜(SEM)对CeO2的物相组成和形貌特征进行表征,分析CeO2粒子的形成过程。将合成的CeO2用于6H-SiC晶片Si面的化学机械抛光(chemical mechanical polishing,CMP),利用原子力显微镜(atomic force microscopy, AFM)和电子天平得出CeO2的抛光性能。结果表明:Ce3+浓度为0.10 mol/L,醇-水体积比为3∶1时合成的CeO2的形貌规则、晶粒尺寸适中且粒度分布均匀。采用其抛光后,晶片表面粗糙度Ra为0.243 nm,材料去除速率dMRR为287 nm/h。合成的CeO2适用于化学机械抛光。Abstract: To obtain homogeneous submicron cerium dioxide (CeO2) polishing powder, CeO2 was synthesized by solvothermal reaction using cerium nitrate (Ce(NO3)3·6H2O) as cerium resource and alcohol-water mixed solution as solvent. The phase composition and morphology of CeO2 were characterized by X-ray diffraction (XRD), laser particle size analyzer and scanning electron microscope (SEM). The formation process of CeO2 particles was analyzed by changing the concentration of Ce3+ and alcohol-water ratio. The synthesized CeO2 was used for chemical mechanical polishing (CMP) Si-face of 6H-SiC. The polishing characteristics were tracked by atomic force microscopy (AFM) and electronic balance. The results indicates that when the Ce3+ concentration is 0.10 mol/L and alcohol/water volume ratio is 3∶1, the as-prepared particles have regular morphology, moderate particle size and uniform particle size distribution. After polished by using the as-prepared CeO2, the Ra of wafer surface reached 0.243 nm and the dMRR 287 nm/h. The as-prepared CeO2 can be used for chemical mechanical polishing.
-
Key words:
- CeO2 /
- homogeneous /
- solvothermal method /
- chemical mechanical polishing
-
表 1 不同CeO2的半高宽、衍射角和晶粒尺寸
Table 1. FWHM, diffraction angle and crystallite size of different CeO2
样品 半高宽
θFWHM / rad衍射角
2θ / rad晶粒尺寸
D / nmCeO2-0.05 0.006 0.248 24 CeO2-0.10 0.008 0.249 18 CeO2-0.15 0.009 0.249 15 -
[1] MIGANI A, VAYSSILOY G N, BROMLEY S T, et al. Greatly facilitated oxygen vacancy formation in ceria nanocrystallites [J]. Chemical Communications,2010,46(32):5936-5938. [2] 耿九光, 臧文杰, 李毅, 等. 纳米二氧化铈的制备及光催化性能 [J]. 化工进展,2014,33(3):720-723. doi: 10.3969/j.issn.1000-6613.2014.03.034GENG Jiuguang, ZANG Wenjie, LI Yi, et al. Preparation and photocatalytic performance of nano-ceria [J]. Chemical Industry and Engineering Progress,2014,33(3):720-723. doi: 10.3969/j.issn.1000-6613.2014.03.034 [3] 王延泽. 晶界对二氧化铈中氧离子自扩散及导电性能的影响 [D]. 秦皇岛: 燕山大学, 2020.WANG Yanze. The effect of grain boundary on self-diffusion and conductivity of oxygen ions in cerium dioxide [D]. Qinhuangdao: Yanshan University, 2020. [4] CHEN G, NI Z, XU L, et al. Performance of colloidal silica and ceria based slurries on CMP of Si-face 6H-SiC substrates [J]. Applied Surface Science,2015,359:664-668. doi: 10.1016/j.apsusc.2015.10.158 [5] 李静, 常民民, 孙明艳, 等. 水热-煅烧法制备亚微米CeO2抛光粉 [J]. 中国稀土学报,2016,34(1):44-49. doi: 10.11785/S1000-4343.20160107LI Jing, CHANG Minmin, SUN Mingyan, et al. Preparation of submicron ceria polishing powder via hydrothermal-calcination method [J]. Journal of the Chinese Society of Rare Earths,2016,34(1):44-49. doi: 10.11785/S1000-4343.20160107 [6] 蒋建忠, 赵永武. 一种新型的机械化学抛光模型 [J]. 江南大学学报,2007,6(5):578-582.JIANG Jianzhong, ZHAO Yongwu. A new model for chemical mechanical polishing [J]. Journal of Jiangnan University,2007,6(5):578-582. [7] 赵敏, 刘文杰. 氧化铈抛光粉粒度分级控制研究 [J]. 中国化工贸易,2013,5(8):387. doi: 10.3969/j.issn.1674-5167.2013.08.361ZHAO Min, LIU Wenjie. Study on particle size grading control of cerium oxide polishing powder [J]. China Chemical Trade,2013,5(8):387. doi: 10.3969/j.issn.1674-5167.2013.08.361 [8] TYRPEKL V, MARKOVA P, DOPITA M, et al. Cerium oxalate morphotypes: Synthesis and conversion into nanocrystalline oxide [J]. Inorganic Chemistry,2019,58(15):10111-10118. doi: 10.1021/acs.inorgchem.9b01250 [9] ZHOU C, ZHU D, WANG D, et al. Synthesis and characterization of cerium dioxide nanoparticles obtained by a novel soft mechanochemical method combined with sol-gel method [J]. Nano Brief Reports and Reviews,2016,12(2):1-8. doi: 10.1142/S1793292017500205 [10] 许伟, 林泽慧, 胡鹏飞, 等. 纳米氧化铈的可控合成及其抛光性能的研究 [J]. 山东化工,2020,49(2):30-32. doi: 10.3969/j.issn.1008-021X.2020.02.011XU Wei, LIN Zehui, HU Pengfei, et al. Controllable synthesis and polishing properties of ceria behavior of ceria nanoparticles [J]. Shandong Chemical Industry,2020,49(2):30-32. doi: 10.3969/j.issn.1008-021X.2020.02.011 [11] 郝顺利, 王新, 崔银芳, 等. 纳米粉体制备过程中粒子的团聚及控制方法研究 [J]. 人工晶体学报,2006,35(2):342-346. doi: 10.3969/j.issn.1000-985X.2006.02.031HAO Shunli, WANG Xin, CUI Yinfang, et al. Investigation on the agglomerate mechanism and controlling method in nano-particle powder preparation [J]. Journal of Synthetic Crystals,2006,35(2):342-346. doi: 10.3969/j.issn.1000-985X.2006.02.031 [12] CHEN H, CHANG H. Homogeneous precipitation of cerium dioxide nanoparticles in alcohol/water mixed solvents [J]. Colloids & Surfaces A Physicochemical & Engineering Aspects,2004,242(1/2/3):61-69. [13] ZHOU F, ZHAO X, XU H, et al. CeO2 spherical crystallites: Synthesis, formation mechanism, size control, and electrochemical property study [J]. The Journal of Physical Chemistry C,2007,111(4):1651-1657. doi: 10.1021/jp0660435 [14] 田皓, 崔建国, 王荣, 等. 强化沉淀反应结晶过程制备纳米稀土氧化物的工艺开发 [J]. 中国稀土学报,2020,186(4):467-473.TIAN Hao, CUI Jianguo, WANG Rong, et al. Technology development of preparation of nanometer rare earth oxides by chemical enhanced process in precipitation crystallization [J]. Journal of the Chinese Society of Rare Earths,2020,186(4):467-473. [15] 李向果, 郭淼, 姚会敏, 等. 菱形二氧化铈的水热合成及发光机理研究 [J]. 无机盐工业,2020,52(6):30-35. doi: 10.11962/1006-4990.2019-0407LI Xiangguo, GUO Miao, YAO Huimin, et al. Research on hydrothermal synthesis and photoluminescence mechanism of rhombus CeO2 [J]. Inorganic Chemical Industry,2020,52(6):30-35. doi: 10.11962/1006-4990.2019-0407 -




 下载:
下载:




 邮件订阅
邮件订阅 RSS
RSS
