| [1] |
国家统计局环境保护部. 中国环境统计年鉴—2016 [M]. 北京: 中国统计出版社, 2018: 4-5.National Bureau of Statistics Ministry of Environmental Protection. China statistical yearbook on environment—2016 [M]. Beijing: China Statistics Press, 2018: 4-5.
|
| [2] |
宋怡明, 徐少伟, 宋昊, 等. 高级氧化法污水处理技术综述 [J]. 山东化工,2019,48(24):211-213. doi: 10.3969/j.issn.1008-021X.2019.24.102SONG Yiming, XU Shaowei, SONG Hao, et al. Summary of advanced oxidation wastewater treatment technology [J]. Shangdong Chemical Industry,2019,48(24):211-213. doi: 10.3969/j.issn.1008-021X.2019.24.102
|
| [3] |
YANG G J, QU X L, SHEN M, et al. Preparation of glassy carbon electrode modified by hydrophobic gold nanoparticles and its application for the determination of ethamsylate in the presence of cetyltrimethylammonium bromide [J]. Sensors and Actuators B: Chemical,2007,128(1):258-265.
|
| [4] |
闫俊娟, 高璟, 刘有智, 等. RuO2–IrO2–SnO/Ti电极电催化氧化降解苯酚废水 [J]. 含能材料,2017,25(9):780-785. doi: 10.11943/j.issn.1006-9941.2017.09.014YAN Junjuan, GAO Jing, LIU Youzhi, et al. Electrocatalytic oxidation degradation of phenol wastewater with RuO2–IrO2–SnO2/Ti anode [J]. Chinese Journal of Energetic Materials,2017,25(9):780-785. doi: 10.11943/j.issn.1006-9941.2017.09.014
|
| [5] |
席耀辉. 掺硼金刚石薄膜的制备、修饰及应用性能研究 [D]. 郑州: 郑州大学, 2017.XI Yaohui. Study on the preparation, modification and application of boron-doped diamond film [D]. Zhengzhou: Zhengzhou University, 2017
|
| [6] |
焦旭阳, 张新妙, 栾金义. 电催化氧化技术处理含盐有机废水研究进展 [J]. 化工环保,2019,39(1):6-10. doi: 10.3969/j.issn.1006-1878.2019.01.002JIAO Xuyang, ZHANG Xinmiao, LUAN Jinyi. Progresses in treatment of salt-containing organic wastewater by electro-catalytic oxidation technology [J]. Environmental Protection of Chemical Industry,2019,39(1):6-10. doi: 10.3969/j.issn.1006-1878.2019.01.002
|
| [7] |
张珂皓. 掺硼金刚石复合电极材料的制备及性能研究 [D]. 郑州: 郑州大学, 2020.ZHANG Kehao. Preparation and properties of boron doped diamond composite electrode materials [D]. Zhengzhou: Zhengzhou University, 2020.
|
| [8] |
闫建明, 徐帅, 康世豪, 等. 掺硼金刚石薄膜的制备与研究 [J]. 超硬材料工程,2019,31(6):1-5.YAN Jianming, XU Shuai, KANG Shihao, et al. Preparation and study of boron-doped diamond films [J]. Superhard Material Engineering,2019,31(6):1-5.
|
| [9] |
POLCARO A M, MASCIA M, PALMAS S, et al. Electrochemical degradation of diuron and dichloroaniline at BDD electrode [J]. Electrochimica Acta,2004,49(4):649-656. doi: 10.1016/j.electacta.2003.09.021
|
| [10] |
马玉祥. 掺硼金刚石膜材料的修饰及应用研究 [D]. 郑州: 郑州大学, 2020.MA Yuxiang. Study on modification and application of boron-doped diamond films [D]. Zhengzhou: Zhengzhou University, 2020.
|
| [11] |
李婧, 柴涛. 电化学氧化法处理工业废水综述 [J]. 广州化工,2012,40(15):46-47,51. doi: 10.3969/j.issn.1001-9677.2012.15.019LI Jing, CHAI Tao. Review of electrochemical oxidation processes applied in industrial wastewater treatment [J]. GuangZhou Chemical Industry,2012,40(15):46-47,51. doi: 10.3969/j.issn.1001-9677.2012.15.019
|
| [12] |
KALISHI R. Doping diamond for electronic applications [J]. Israel Journal of Chemistry,1998,38(1/2):41-50. doi: 10.1002/ijch.199800005
|
| [13] |
SHAKHOV F M, ABYZOV A M, TAKAI K. Boron doped diamond synthesized from detonation nanodiamond in a C-O-H fluid at high pressure and high temperature [J]. Journal of Solid State Chemistry,2017,256:72-92. doi: 10.1016/j.jssc.2017.08.009
|
| [14] |
DUBROVINSKAIA N, DUBROVINSKY L, MIYAJIMA N, et al. High-pressure/high-temperature synthesis and characterization of boron-doped diamond [J]. Zeitschrift für Naturforschung B,2006,61(12):1561-1565.
|
| [15] |
ZAHNG K H, WANG H L, SHAO G, et al. Preparation and properties of boron-doped diamond composites fabricated by high-pressure and high-temperature sintering [J]. Ceramics International,2019,45(7):9271-9277. doi: 10.1016/j.ceramint.2019.02.005
|
| [16] |
ZHU C W, JIANG C Q, CHEN S, et al. Ultrasound enhanced electrochemical oxidation of Alizarin Red S on boron doped diamond(BDD) anode: Effect of degradation process parameters [J]. Chemosphere,2018,209:685-695.
|
| [17] |
HE Y P, LIN H B, GUO Z C, et al. Recent developments and advances in boron-doped diamond electrodes for electrochemical oxidation of organic pollutants [J]. Separation and Purification Technology,2019,212(1):802-821.
|
| [18] |
HE Y P, HUANG W M, CHEN R L, et al. Improved electrochemical performance of boron-doped diamond electrode depending on the structure of titanium substrate [J]. Journal of Electroanalytical Chemistry,2015,758(1):170-177.
|
| [19] |
MEI R Q, WEI Q P, ZHU C W, et al. 3D macroporous boron-doped diamond electrode with interconnected liquid flow channels: A high-efficiency electrochemical degradation of RB-19 dye wastewater under low current [J]. Applied Catalysis B: Environmental,2019,245:420-427. doi: 10.1016/j.apcatb.2018.12.074
|
| [20] |
SUO N, HUANG H, WU A M, et al. Porous boron doped diamonds as metal-free catalysts for the oxygen reduction reaction in alkaline solution [J]. Applied Surface Science,2018,439(1):329-335.
|
| [21] |
HE Y P, LIN H B, WANG X, et al. A hydrophobic three-dimensionally networked boron-doped diamond electrode towards electrochemical oxidation [J]. Chemical Communications,2016,52(51):8026-8029. doi: 10.1039/C6CC03866B
|
| [22] |
CHAPLIN B P, WYLE I, ZENG H J, et al. Characterization of the performance and failure mechanisms of boron-doped ultrananocrystalline diamond electrodes [J]. Journal of Applied Electrochemistry,2011,41(11):1329-1340. doi: 10.1007/s10800-011-0351-7
|
| [23] |
PETRÁK V, ŽIVCOVÁ Z, KRYSOVA H, et al. Fabrication of porous boron-doped diamond on SiO2 fiber templates [J]. Carbon,2017,114:457-464. doi: 10.1016/j.carbon.2016.12.012
|
| [24] |
LEE C H, LEE E S, LIM Y K, et al. Enhanced electrochemical oxidation of phenol by boron-doped diamond nanowire electrode [J]. RSC Advances,2017,7:6229-6235. doi: 10.1039/C6RA26287B
|
| [25] |
LEE S K, SONG M J, LIM D S. Morphology control of 3D-networked boron-doped diamond nanowires and its electrochemical properties [J]. Journal of Electroanalytical Chemistry,2018,820:140-145. doi: 10.1016/j.jelechem.2018.04.056
|
| [26] |
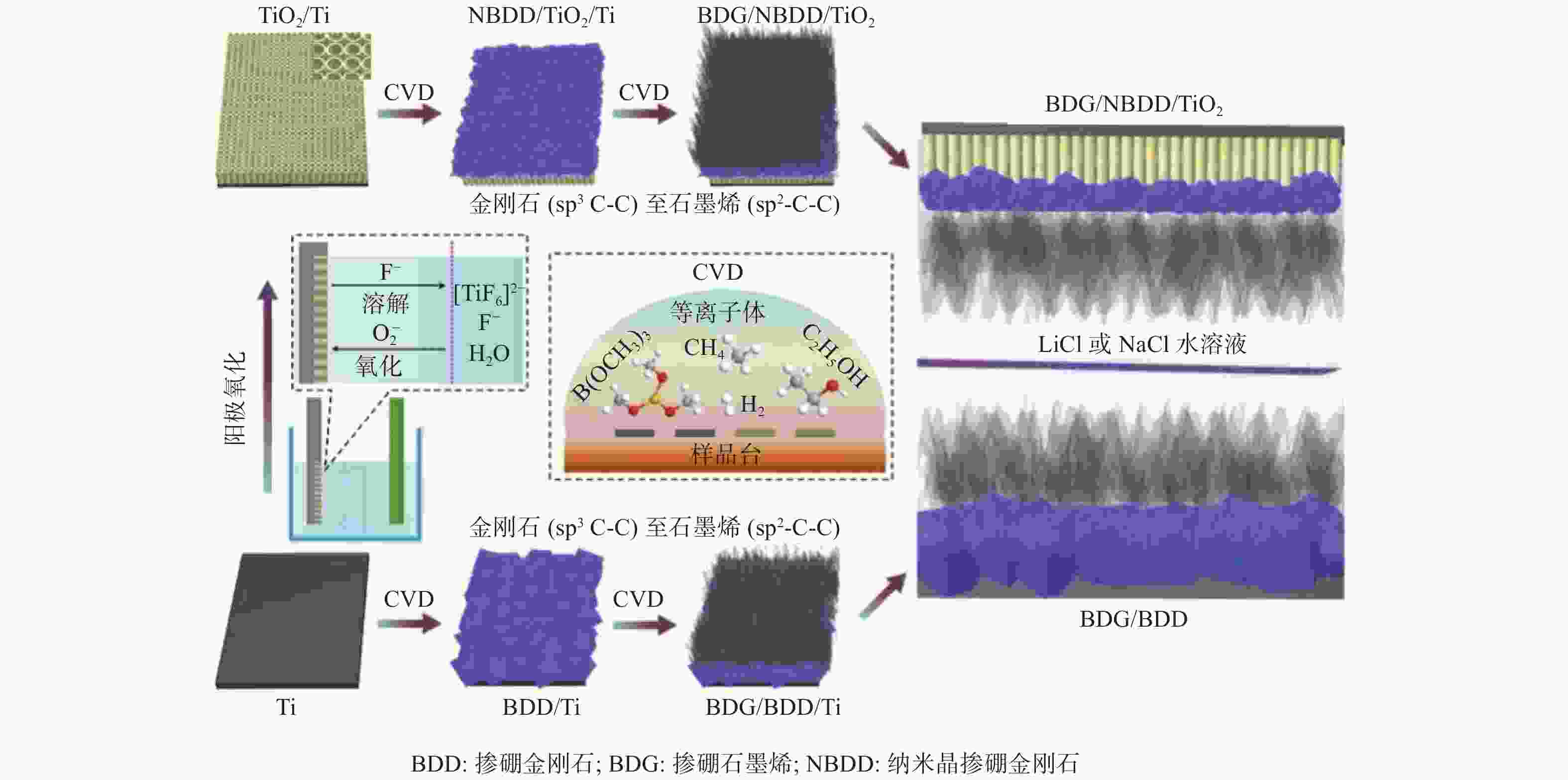
YAN W K, LI M J, LI H J, et al. Aqueous lithium and sodium ion capacitors with boron-doped graphene/BDD/TiO2 anode and boron-doped graphene/BDD cathode exhibiting AC line-filtering performance [J]. Chemical Engineering Journal,2020,388(15):124265.
|
| [27] |
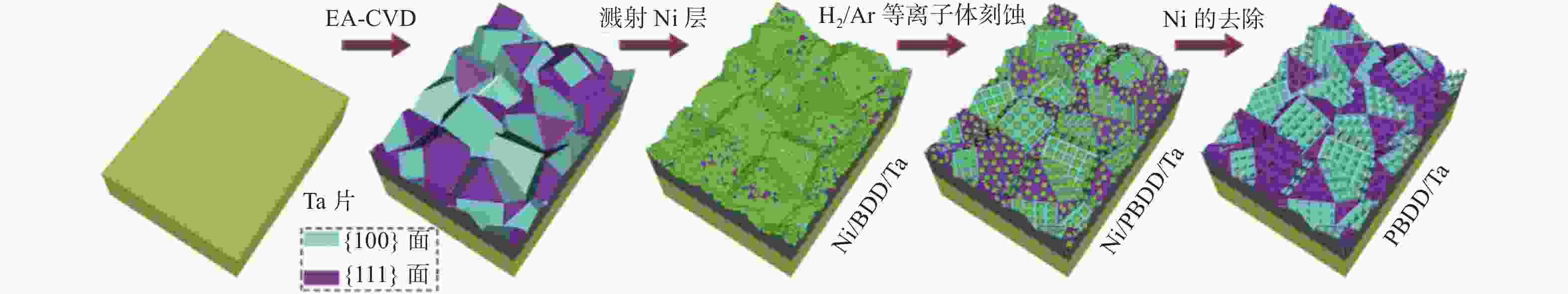
LI X J, LI H J, LI M J, et al. Preparation of a porous boron-doped diamond/Ta electrode for the electrocatalytic degradation of organic pollutants [J]. Carbon,2018,129:543-551. doi: 10.1016/j.carbon.2017.12.052
|
| [28] |
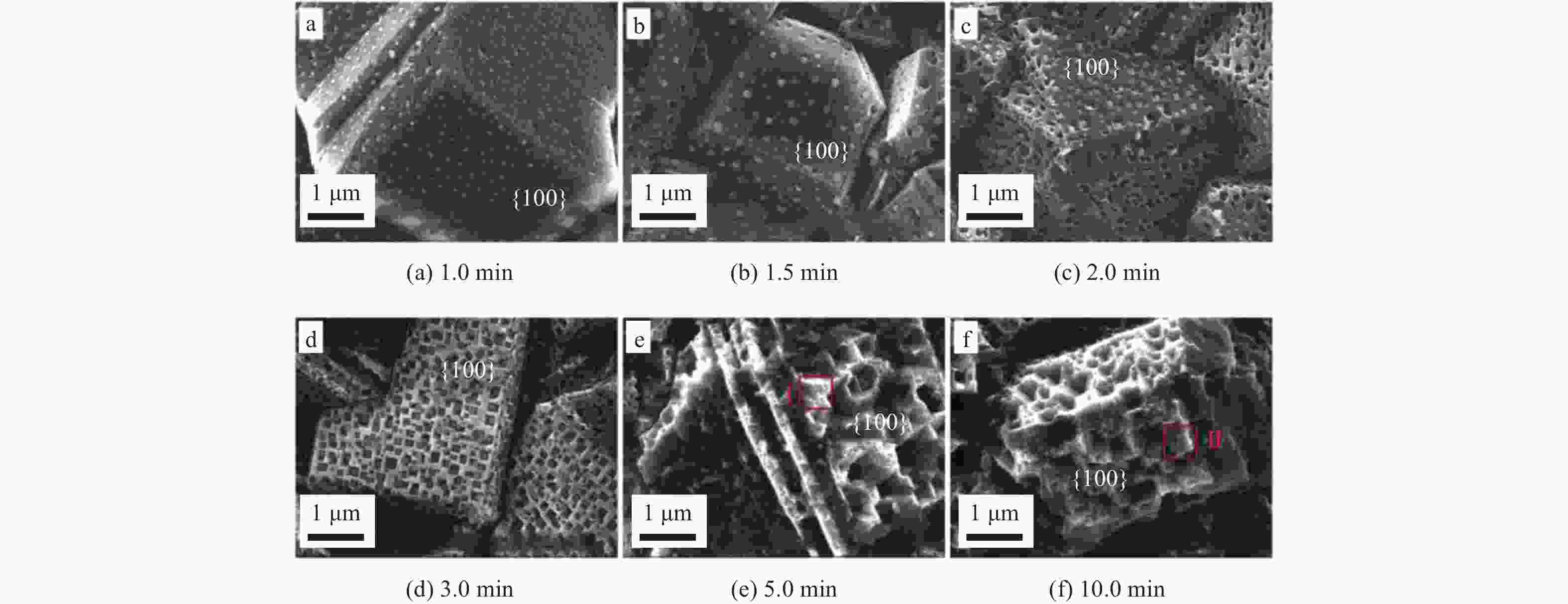
MIAO D T, LIU T, YU Y L, et al. Study on degradation performance and stability of high temperature etching boron-doped diamond electrode [J]. Applied Surface Science,2020,514:146091. doi: 10.1016/j.apsusc.2020.146091
|
| [29] |
ZHUO Q F, WANG J B, NIU J F, et al. Electrochemical oxidation of perfluorooctane sulfonate (PFOS) substitute by modified boron doped diamond (BDD) anodes [J]. Chemical Engineering Journal,2020,379:122280. doi: 10.1016/j.cej.2019.122280
|
| [30] |
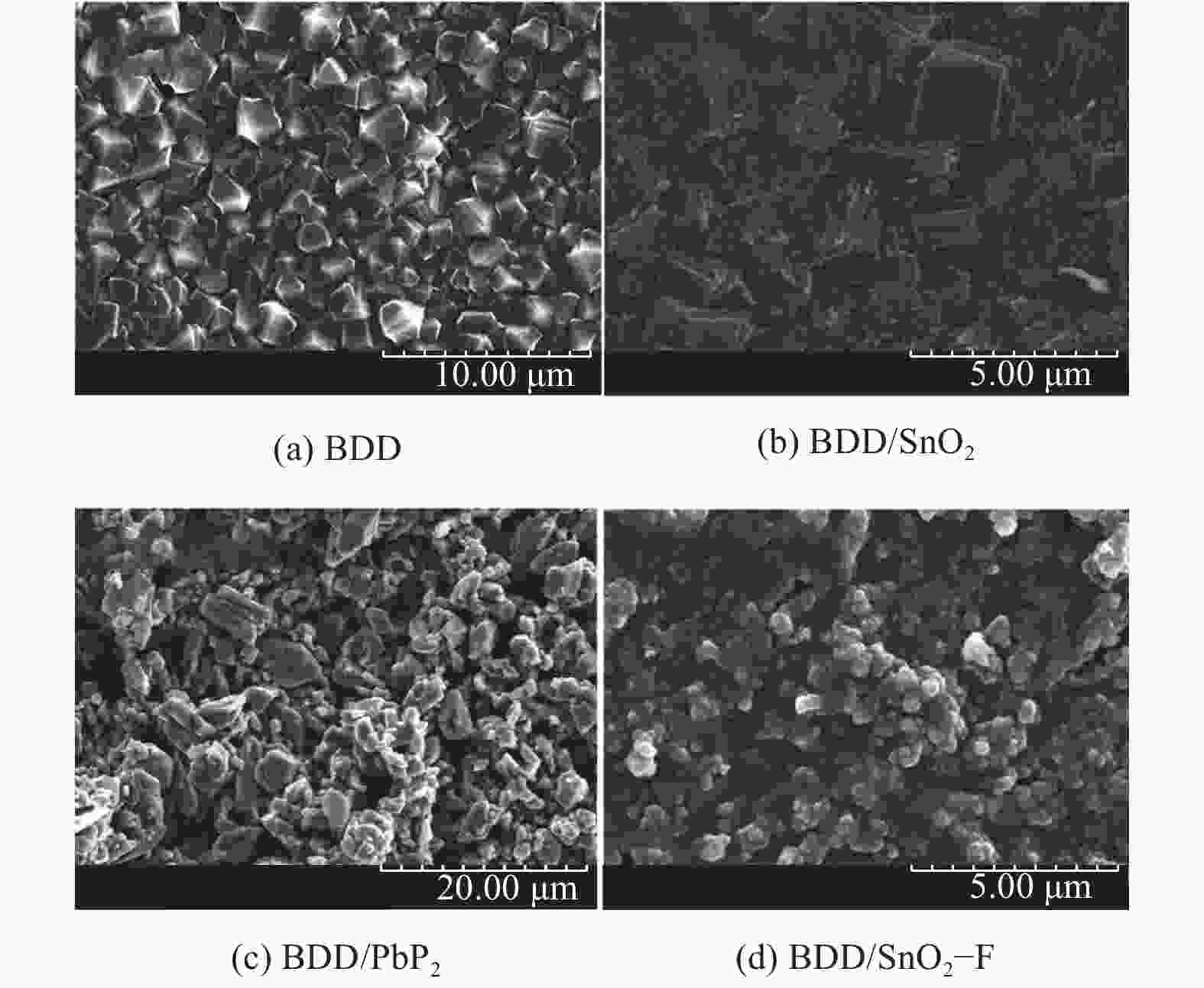
席耀辉, 王海龙, 闫宁, 等. TiO2修饰BDD复合电极材料的制备及性能 [J]. 硅酸盐学报,2018,46(3):322-327.XI Yaohui, WANG Hailong, YAN Ning, et al. Preparation and properties of TiO2 modified BDD composite electrode [J]. Journal of the Chinese Ceramic Society,2018,46(3):322-327.
|




 下载:
下载:


 邮件订阅
邮件订阅 RSS
RSS
